Selective chemical genetic inhibition of protein kinase C epsilon reduces ethanol consumption in mice
- PMID: 26947945
- PMCID: PMC4912951
- DOI: 10.1016/j.neuropharm.2016.02.036
Selective chemical genetic inhibition of protein kinase C epsilon reduces ethanol consumption in mice
Abstract
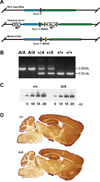
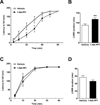
Reducing expression or inhibiting translocation of protein kinase C epsilon (PKCε) prolongs ethanol intoxication and decreases ethanol consumption in mice. However, we do not know if this phenotype is due to reduced PKCε kinase activity or to impairment of kinase-independent functions. In this study, we used a chemical-genetic strategy to determine whether a potent and highly selective inhibitor of PKCε catalytic activity reduces ethanol consumption. We generated ATP analog-specific PKCε (AS-PKCε) knock-in mice harboring a point mutation in the ATP binding site of PKCε that renders the mutant kinase highly sensitive to inhibition by 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine (1-NA-PP1). Systemically administered 1-NA-PP1 readily crossed the blood brain barrier and inhibited PKCε-mediated phosphorylation. 1-NA-PP1 reversibly reduced ethanol consumption by AS-PKCε mice but not by wild type mice lacking the AS-PKCε mutation. These results support the development of inhibitors of PKCε catalytic activity as a strategy to reduce ethanol consumption, and they demonstrate that the AS- PKCε mouse is a useful tool to study the role of PKCε in behavior.
Keywords: 1-NA-PP1; 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine (PubChem CID: 4877); AS-Kinase; Alcohol; Ethanol; Protein kinase C.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
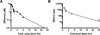



References
-
- Bishop AC, Buzko O, Shokat KM. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–172. - PubMed
-
- Cozzoli DK, Courson J, Rostock C, Campbell RR, Wroten MG, McGregor H, Caruana AL, Miller BW, Hu JH, Wu Zhang P, Xiao B, Worley PF, Crabbe JC, Finn DA, Szumlinski KK. Protein kinase C epsilon activity in the nucleus accumbens and central nucleus of the amygdala mediates binge alcohol consumption. Biol Psychiatry. 2015 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

