Leveraging gene-environment interactions and endotypes for asthma gene discovery
- PMID: 26947980
- PMCID: PMC5004762
- DOI: 10.1016/j.jaci.2016.01.006
Leveraging gene-environment interactions and endotypes for asthma gene discovery
Abstract
Asthma is a heterogeneous clinical syndrome that includes subtypes of disease with different underlying causes and disease mechanisms. Asthma is caused by a complex interaction between genes and environmental exposures; early-life exposures in particular play an important role. Asthma is also heritable, and a number of susceptibility variants have been discovered in genome-wide association studies, although the known risk alleles explain only a small proportion of the heritability. In this review, we present evidence supporting the hypothesis that focusing on more specific asthma phenotypes, such as childhood asthma with severe exacerbations, and on relevant exposures that are involved in gene-environment interactions (GEIs), such as rhinovirus infections, will improve detection of asthma genes and our understanding of the underlying mechanisms. We will discuss the challenges of considering GEIs and the advantages of studying responses to asthma-associated exposures in clinical birth cohorts, as well as in cell models of GEIs, to dissect the context-specific nature of genotypic risks, to prioritize variants in genome-wide association studies, and to identify pathways involved in pathogenesis in subgroups of patients. We propose that such approaches, in spite of their many challenges, present great opportunities for better understanding of asthma pathogenesis and heterogeneity and, ultimately, for improving prevention and treatment of disease.
Keywords: 17q asthma locus; Asthma; CDHR3; gene-environment interactions; genome-wide association study; rhinovirus.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interestC. Ober has received grants from the National Institutes of Health. K. Bønnelykke declares that he has no relevant conflicts of interest.
Figures

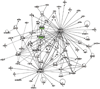
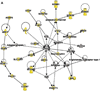

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

