Hyperoside induces both autophagy and apoptosis in non-small cell lung cancer cells in vitro
- PMID: 26948085
- PMCID: PMC4820797
- DOI: 10.1038/aps.2015.148
Hyperoside induces both autophagy and apoptosis in non-small cell lung cancer cells in vitro
Abstract
Aim: Hyperoside (quercetin-3-O-β-D-galactopyranoside) is a flavonol glycoside found in plants of the genera Hypericum and Crataegus, which exhibits anticancer, anti-oxidant, and anti-inflammatory activities. In this study we investigated whether autophagy was involved in the anticancer mechanisms of hyperoside in human non-small cell lung cancer cells in vitro.
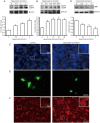
Methods: Human non-small cell lung cancer cell line A549 was tested, and human bronchial epithelial cell line BEAS-2B was used for comparison. The expression of LC3-II, apoptotic and signaling proteins was measured using Western blotting. Autophagosomes were observed with MDC staining, LC3 immunocytochemistry, and GFP-LC3 fusion protein techniques. Cell viability was assessed using MTT assay.
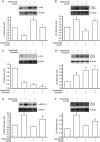
Results: Hyperoside (0.5, 1, 2 mmol/L) dose-dependently increased the expression of LC3-II and autophagosome numbers in A549 cells, but had no such effects in BEAS-2B cells. Moreover, hyperoside dose-dependently inhibited the phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, but increased the phosphorylation of ERK1/2 in A549 cells. Insulin (200 nmol/L) markedly enhanced the phosphorylation of Akt and decreased LC3-II expression in A549 cells, which were reversed by pretreatment with hyperoside, whereas the MEK1/2 inhibitor U0126 (20 μmol/L) did not blocked hyperoside-induced LC3-II expression. Finally, hyperoside dose-dependently suppressed the cell viability and induced apoptosis in A549 cells, which were significantly attenuated by pretreatment with the autophagy inhibitor 3-methyladenine (2.5 mmol/L).
Conclusion: Hyperoside induces both autophagy and apoptosis in human non-small cell lung cancer cells in vitro. The autophagy is induced through inhibiting the Akt/mTOR/p70S6K signal pathways, which contributes to anticancer actions of hyperoside.
Figures






Similar articles
-
7-O-geranylquercetin-induced autophagy contributes to apoptosis via ROS generation in human non-small cell lung cancer cells.Life Sci. 2017 Jul 1;180:102-113. doi: 10.1016/j.lfs.2017.05.008. Epub 2017 May 8. Life Sci. 2017. PMID: 28495516
-
Resveratrol analogue 3,4,4'-trihydroxy-trans-stilbene induces apoptosis and autophagy in human non-small-cell lung cancer cells in vitro.Acta Pharmacol Sin. 2015 Oct;36(10):1256-65. doi: 10.1038/aps.2015.46. Epub 2015 Jul 20. Acta Pharmacol Sin. 2015. PMID: 26190500 Free PMC article.
-
Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):263-269. doi: 10.1016/j.bbrc.2017.10.042. Epub 2017 Oct 9. Biochem Biophys Res Commun. 2017. PMID: 29024631
-
Inhibition of autophagy enhances heat-induced apoptosis in human non-small cell lung cancer cells through ER stress pathways.Arch Biochem Biophys. 2016 Oct 1;607:55-66. doi: 10.1016/j.abb.2016.08.016. Epub 2016 Aug 24. Arch Biochem Biophys. 2016. PMID: 27565443 Review.
-
Hyperoside: A review on its sources, biological activities, and molecular mechanisms.Phytother Res. 2022 Jul;36(7):2779-2802. doi: 10.1002/ptr.7478. Epub 2022 May 13. Phytother Res. 2022. PMID: 35561084 Review.
Cited by
-
Hypericum Genus as a Natural Source for Biologically Active Compounds.Plants (Basel). 2022 Sep 26;11(19):2509. doi: 10.3390/plants11192509. Plants (Basel). 2022. PMID: 36235373 Free PMC article. Review.
-
Polyphenol-Mediated Autophagy in Cancer: Evidence of In Vitro and In Vivo Studies.Int J Mol Sci. 2020 Sep 10;21(18):6635. doi: 10.3390/ijms21186635. Int J Mol Sci. 2020. PMID: 32927836 Free PMC article. Review.
-
Hyperoside Protects Human Umbilical Vein Endothelial Cells Against Anticardiolipin Antibody-Induced Injury by Activating Autophagy.Front Pharmacol. 2020 May 21;11:762. doi: 10.3389/fphar.2020.00762. eCollection 2020. Front Pharmacol. 2020. PMID: 32508661 Free PMC article.
-
The Biopotential of Bellardia trixago in Replacing Synthetic Compounds for Health-Promoting Applications: Is It a Promising Candidate?Food Sci Nutr. 2025 Apr 14;13(4):e70109. doi: 10.1002/fsn3.70109. eCollection 2025 Apr. Food Sci Nutr. 2025. PMID: 40236829 Free PMC article.
-
Study on the mechanism of hyperoside in affecting the biological progression and radiosensitivity of esophageal carcinoma by modulating the STAT3/AKT/ERK pathway.Biomol Biomed. 2025 Apr 3;25(5):1150-1161. doi: 10.17305/bb.2024.11201. Biomol Biomed. 2025. PMID: 39558814 Free PMC article.
References
-
- Yuan J, Horvitz HR. A first insight into the molecular mechanisms of apoptosis. Cell 2004; 116: S53–6, 1 p following S59. - PubMed
-
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995; 376: 37–43. - PubMed
-
- Farias A, Cabrerizo M, Re V, Glatstein N, Pisano B, Spinsanti L, et al. Molecular identification of human enteroviruses in children with neurological infections from the central region of Argentina. Arch Virol 2011; 156: 129–33. - PubMed
-
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402: 672–6. - PubMed
-
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 2009; 16: 966–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

