Prostate cancer - evidence of exercise and nutrition trial (PrEvENT): study protocol for a randomised controlled feasibility trial
- PMID: 26948468
- PMCID: PMC4780152
- DOI: 10.1186/s13063-016-1248-x
Prostate cancer - evidence of exercise and nutrition trial (PrEvENT): study protocol for a randomised controlled feasibility trial
Abstract
Background: A growing body of observational evidence suggests that nutritional and physical activity interventions are associated with beneficial outcomes for men with prostate cancer, including brisk walking, lycopene intake, increased fruit and vegetable intake and reduced dairy consumption. However, randomised controlled trial data are limited. The 'Prostate Cancer: Evidence of Exercise and Nutrition Trial' investigates the feasibility of recruiting and randomising men diagnosed with localised prostate cancer and eligible for radical prostatectomy to interventions that modify nutrition and physical activity. The primary outcomes are randomisation rates and adherence to the interventions at 6 months following randomisation. The secondary outcomes are intervention tolerability, trial retention, change in prostate specific antigen level, change in diet, change in general physical activity levels, insulin-like growth factor levels, and a range of related outcomes, including quality of life measures.
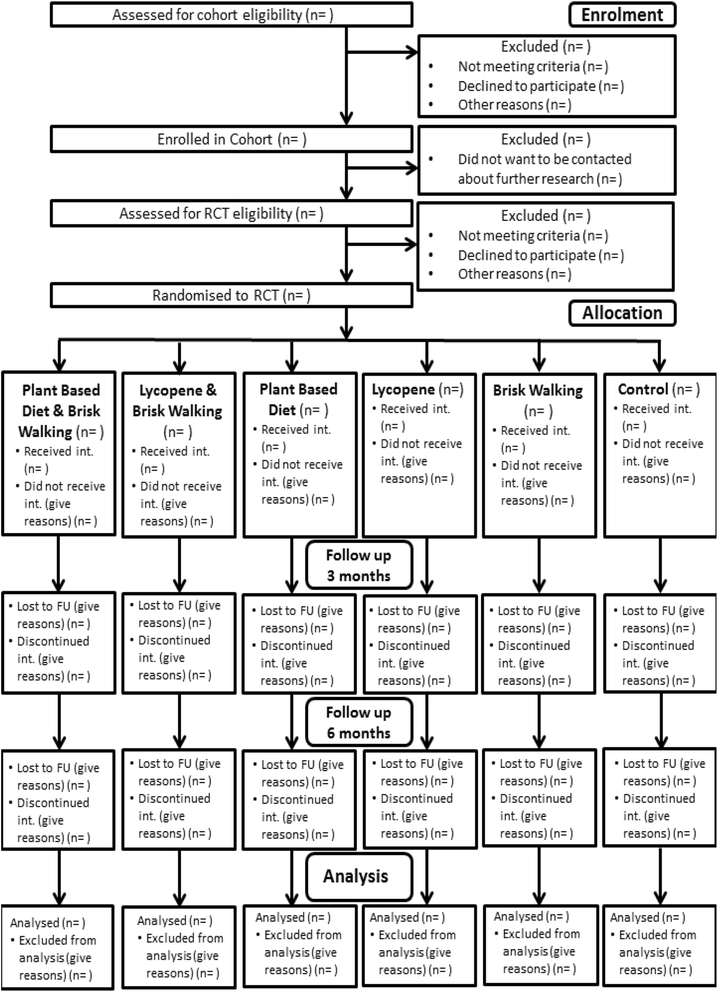
Methods/design: The trial is factorial, randomising men to both a physical activity (brisk walking or control) and nutritional (lycopene supplementation or increased fruit and vegetables with reduced dairy consumption or control) intervention. The trial has two phases: men are enrolled into a cohort study prior to radical prostatectomy, and then consented after radical prostatectomy into a randomised controlled trial. Data are collected at four time points (cohort baseline, true trial baseline and 3 and 6 months post-randomisation).
Discussion: The Prostate Cancer: Evidence of Exercise and Nutrition Trial aims to determine whether men with localised prostate cancer who are scheduled for radical prostatectomy can be recruited into a cohort and subsequently randomised to a 6-month nutrition and physical activity intervention trial. If successful, this feasibility trial will inform a larger trial to investigate whether this population will gain clinical benefit from long-term nutritional and physical activity interventions post-surgery. Prostate Cancer: Evidence of Exercise and Nutrition Trial (PrEvENT) is registered on the ISRCTN registry, ref number ISRCTN99048944. Date of registration 17 November 2014.
Figures
References
-
- Cancer Research UK. Prostate Cancer Key Facts. 2013.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical