Catch-bond mechanism of the bacterial adhesin FimH
- PMID: 26948702
- PMCID: PMC4786642
- DOI: 10.1038/ncomms10738
Catch-bond mechanism of the bacterial adhesin FimH
Abstract
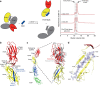
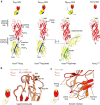
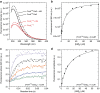
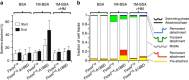
Ligand-receptor interactions that are reinforced by mechanical stress, so-called catch-bonds, play a major role in cell-cell adhesion. They critically contribute to widespread urinary tract infections by pathogenic Escherichia coli strains. These pathogens attach to host epithelia via the adhesin FimH, a two-domain protein at the tip of type I pili recognizing terminal mannoses on epithelial glycoproteins. Here we establish peptide-complemented FimH as a model system for fimbrial FimH function. We reveal a three-state mechanism of FimH catch-bond formation based on crystal structures of all states, kinetic analysis of ligand interaction and molecular dynamics simulations. In the absence of tensile force, the FimH pilin domain allosterically accelerates spontaneous ligand dissociation from the FimH lectin domain by 100,000-fold, resulting in weak affinity. Separation of the FimH domains under stress abolishes allosteric interplay and increases the affinity of the lectin domain. Cell tracking demonstrates that rapid ligand dissociation from FimH supports motility of piliated E. coli on mannosylated surfaces in the absence of shear force.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

