1,25(OH)2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis)
- PMID: 26949104
- PMCID: PMC4825694
- DOI: 10.1016/j.jsbmb.2016.03.002
1,25(OH)2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis)
Abstract
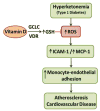
Background: There is a significantly higher incidence of cardiovascular disease (CVD) among type 1 diabetic (T1D) patients than among non-diabetic subjects. T1D is associated with hyperketonemia, a condition with elevated blood levels of ketones, in addition to hyperglycemia. The biochemical mechanism by which vitamin D (VD) may reduce the risk of CVD is not known. This study examines whether VD can be beneficial in reducing hyperketonemia (acetoacetate, AA) induced oxidative stress in endothelial cells.
Methods: HUVEC were pretreated with 1,25(OH)2D3, and later exposed to the ketone body acetoacetate.
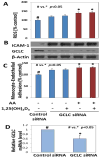
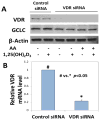
Results: The increases in ROS production, ICAM-1 expression, MCP-1 secretion, and monocyte adhesion in HUVEC treated with AA were significantly reduced following treatment with 1,25(OH)2D3. Interestingly, an increase in glutathione (GSH) levels was also observed with 1,25(OH)2D3 in ketone treated cells. The effects of 1,25(OH)2D3 on GSH, ROS, and monocyte-endothelial adhesion were prevented in GCLC knockdown HUVEC. This suggests that 1,25(OH)2D3 inhibits ROS, MCP-1, ICAM-1, and adherence of monocytes mediated by the upregulation of GCLC and GSH.
Conclusion: This study provides evidence for the biochemical mechanism through which VD supplementation may reduce the excess monocyte adhesion to endothelium and inflammation associated with T1D.
Keywords: 1,25(OH)(2)D3; Endothelium; Ketones; Oxidative stress; Type 1 diabetes; Vitamin D.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Authors declare they have no conflicts of interests.
Figures



References
-
- Daly RM, Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Sikaris KA, Zimmet PZ, Ebeling PR, Shaw JE. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf) 2012;77:26–35. - PubMed
-
- Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni OR, Hadioonzadeh R, Bhandari M. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45:365–378. - PubMed
-
- Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. - PubMed
-
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. - PubMed
-
- Alvarez MM, Reiff e Vieira AC, Luiz RR, da Veiga GV. Validation of capillary glycemia as a strategy for the screening of diabetes mellitus in adolescents. Pediatr Diabetes. 2009;10:449–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

