BOK Is a Non-canonical BCL-2 Family Effector of Apoptosis Regulated by ER-Associated Degradation
- PMID: 26949185
- PMCID: PMC5018360
- DOI: 10.1016/j.cell.2016.02.026
BOK Is a Non-canonical BCL-2 Family Effector of Apoptosis Regulated by ER-Associated Degradation
Abstract
The mitochondrial pathway of apoptosis is initiated by mitochondrial outer membrane permeabilization (MOMP). The BCL-2 family effectors BAX and BAK are thought to be absolutely required for this process. Here, we report that BCL-2 ovarian killer (BOK) is a bona fide yet unconventional effector of MOMP that can trigger apoptosis in the absence of both BAX and BAK. However, unlike the canonical effectors, BOK appears to be constitutively active and unresponsive to antagonistic effects of the antiapoptotic BCL-2 proteins. Rather, BOK is controlled at the level of protein stability by components of the endoplasmic reticulum (ER)-associated degradation pathway. BOK is ubiquitylated by the AMFR/gp78 E3 ubiquitin ligase complex and targeted for proteasomal degradation in a VCP/p97-dependent manner, which allows survival of the cell. When proteasome function, VCP, or gp78 activity is compromised, BOK is stabilized to induce MOMP and apoptosis independently of other BCL-2 proteins.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
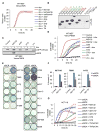
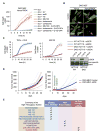
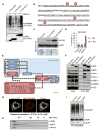
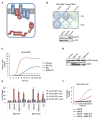
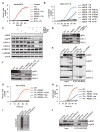
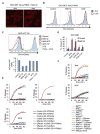
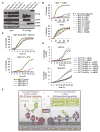
Comment in
-
Cell Biology: ERADicating Survival with BOK.Curr Biol. 2016 Jun 6;26(11):R473-6. doi: 10.1016/j.cub.2016.04.003. Curr Biol. 2016. PMID: 27269726
References
-
- Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

