Midbrain dopamine neurons compute inferred and cached value prediction errors in a common framework
- PMID: 26949249
- PMCID: PMC4805544
- DOI: 10.7554/eLife.13665
Midbrain dopamine neurons compute inferred and cached value prediction errors in a common framework
Abstract
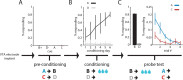
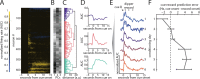
Midbrain dopamine neurons have been proposed to signal reward prediction errors as defined in temporal difference (TD) learning algorithms. While these models have been extremely powerful in interpreting dopamine activity, they typically do not use value derived through inference in computing errors. This is important because much real world behavior - and thus many opportunities for error-driven learning - is based on such predictions. Here, we show that error-signaling rat dopamine neurons respond to the inferred, model-based value of cues that have not been paired with reward and do so in the same framework as they track the putative cached value of cues previously paired with reward. This suggests that dopamine neurons access a wider variety of information than contemplated by standard TD models and that, while their firing conforms to predictions of TD models in some cases, they may not be restricted to signaling errors from TD predictions.
Keywords: dopamine; neuroscience; prediction error; rat; single unit.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Comment in
-
The expanding role of dopamine.Elife. 2016 Apr 21;5:e15963. doi: 10.7554/eLife.15963. Elife. 2016. PMID: 27099987 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

