Cryo-EM single particle analysis with the Volta phase plate
- PMID: 26949259
- PMCID: PMC4850076
- DOI: 10.7554/eLife.13046
Cryo-EM single particle analysis with the Volta phase plate
Abstract
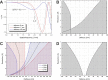
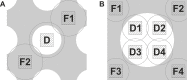
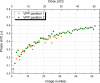
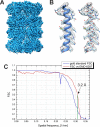
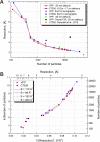
We present a method for in-focus data acquisition with a phase plate that enables near-atomic resolution single particle reconstructions. Accurate focusing is the determining factor for obtaining high quality data. A double-area focusing strategy was implemented in order to achieve the required precision. With this approach we obtained a 3.2 Å resolution reconstruction of the Thermoplasma acidophilum 20S proteasome. The phase plate matches or slightly exceeds the performance of the conventional defocus approach. Spherical aberration becomes a limiting factor for achieving resolutions below 3 Å with in-focus phase plate images. The phase plate could enable single particle analysis of challenging samples in terms of small size, heterogeneity and flexibility that are difficult to solve by the conventional defocus approach.
Keywords: biophysics; cryo-EM; none; phase plate; single particle analysis; structural biology.
Conflict of interest statement
RD: Co-inventor in US patent US9129774 B2 "Method of using a phase plate in a transmission electron microscope".
WB: Scientific Advisory Board of FEI Company.
Figures

Comment in
-
Protein complexes in focus.Elife. 2016 Apr 28;5:e16156. doi: 10.7554/eLife.16156. Elife. 2016. PMID: 27124202 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

