Low Density Lipoprotein Receptor Related Proteins as Regulators of Neural Stem and Progenitor Cell Function
- PMID: 26949399
- PMCID: PMC4754494
- DOI: 10.1155/2016/2108495
Low Density Lipoprotein Receptor Related Proteins as Regulators of Neural Stem and Progenitor Cell Function
Abstract
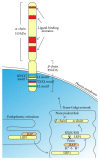
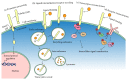
The central nervous system (CNS) is a highly organised structure. Many signalling systems work in concert to ensure that neural stem cells are appropriately directed to generate progenitor cells, which in turn mature into functional cell types including projection neurons, interneurons, astrocytes, and oligodendrocytes. Herein we explore the role of the low density lipoprotein (LDL) receptor family, in particular family members LRP1 and LRP2, in regulating the behaviour of neural stem and progenitor cells during development and adulthood. The ability of LRP1 and LRP2 to bind a diverse and extensive range of ligands, regulate ligand endocytosis, recruit nonreceptor tyrosine kinases for direct signal transduction and signal in conjunction with other receptors, enables them to modulate many crucial neural cell functions.
Figures
References
-
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. - PubMed
-
- Binder R. J., Han D. K., Srivastava P. K. CD91: a receptor for heat shock protein gp96. Nature Immunology. 2000;1(2):151–155. - PubMed
-
- Saito A., Pietromonaco S., Loo A., et al. Complete cloning and sequencing of rat Gp330 megalin, a distinctive member of the low-density-lipoprotein receptor gene family. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9725–9729. doi: 10.1073/pnas.91.21.9725. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

