Buccal injection of synthetic HPV long peptide vaccine induces local and systemic antigen-specific CD8+ T-cell immune responses and antitumor effects without adjuvant
- PMID: 26949512
- PMCID: PMC4778350
- DOI: 10.1186/s13578-016-0083-9
Buccal injection of synthetic HPV long peptide vaccine induces local and systemic antigen-specific CD8+ T-cell immune responses and antitumor effects without adjuvant
Abstract
Background: Human Papillomavirus is responsible for over 99 % of cervical cancers and is associated with cancers of the head and neck. The currently available prophylactic vaccines against HPV do not generate therapeutic effects against established HPV infections and associated lesions and disease. Thus, the need for a therapeutic vaccine capable of treating HPV-induced malignancies persists. Synthetic long peptides vaccination is a popular antigen delivery method because of its safety, stability, production feasibility, and its need to be processed by professional antigen presenting cells before it can be presented to cytotoxic CD8+ T lymphocytes. Cancers in the buccal mucosa have been shown to elicit cancer-related inflammations that are capable of recruiting inflammatory and immune cells to generate antitumor effects. In the current study, we evaluated the therapeutic potential of synthetic HPV long peptide vaccination in the absence of adjuvant in the TC-1 buccal tumor model.
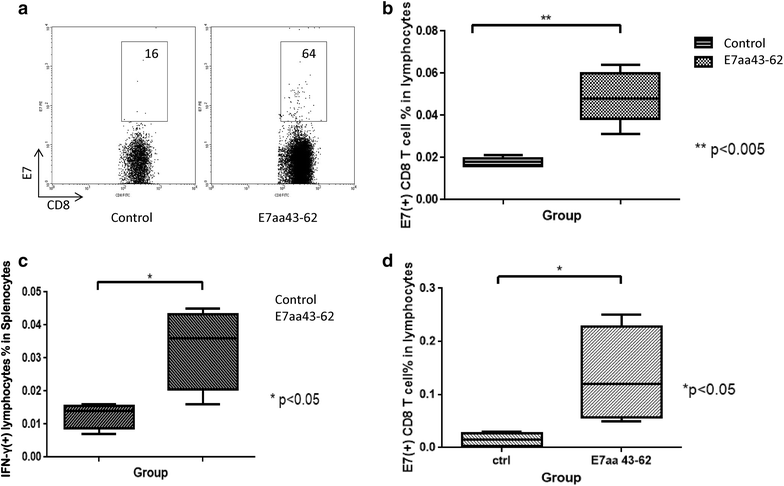
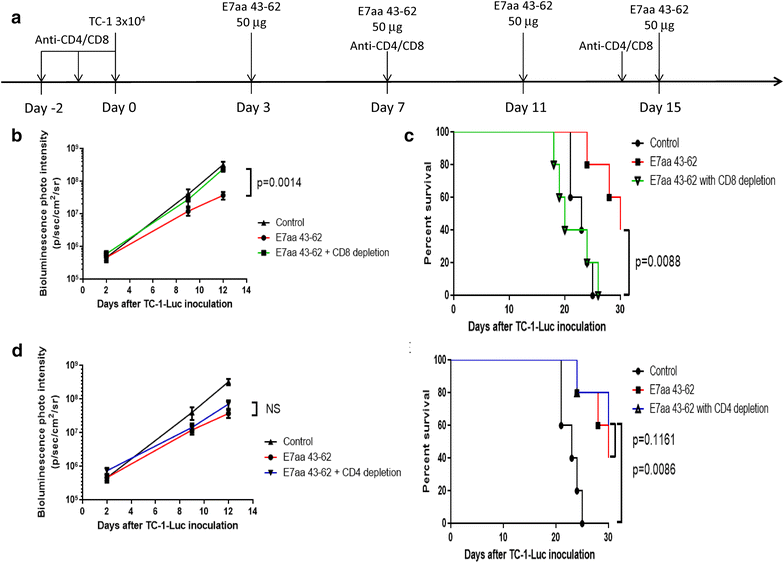
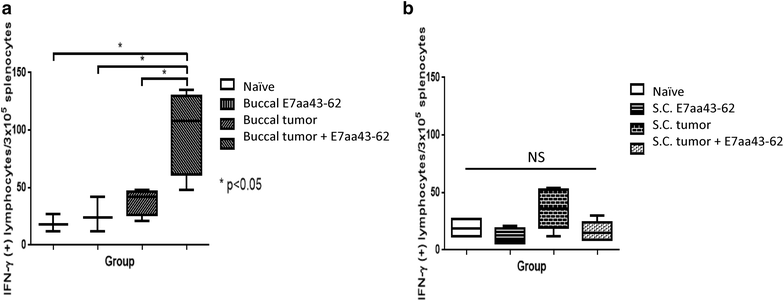
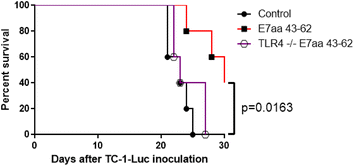
Result: We show that intratumoral vaccination with E7 long peptide alone effectively controls buccal TC-1 tumors in mice. Furthermore, we observed an increase in systemic as well as local E7-specific CD8+ T cells in buccal tumor-bearing mice following the vaccination. Finally, we show that induction of immune responses against buccal tumors by intratumoral E7 long peptide vaccination is independent of CD4+ T cells, and that the phenomenon may be related to the unique environment associated with mucosal tissues.
Conclusion: Our results suggest the possibility for clinical translation of the administration of adjuvant free therapeutic long peptide vaccine as a potentially effective and safe strategy for mucosal HPV-associated tumor treatment.
Keywords: Adjuvant free; Buccal tumor; E7 long peptide; Immunotherapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication.BMC Cancer. 2019 Jun 6;19(1):540. doi: 10.1186/s12885-019-5725-y. BMC Cancer. 2019. PMID: 31170937 Free PMC article.
-
Prevention and treatment of cervical cancer by a single administration of human papillomavirus peptide vaccine with CpG oligodeoxynucleotides as an adjuvant in vivo.Int Immunopharmacol. 2019 Apr;69:279-288. doi: 10.1016/j.intimp.2019.01.024. Epub 2019 Feb 8. Int Immunopharmacol. 2019. PMID: 30743204
-
Local HPV Recombinant Vaccinia Boost Following Priming with an HPV DNA Vaccine Enhances Local HPV-Specific CD8+ T-cell-Mediated Tumor Control in the Genital Tract.Clin Cancer Res. 2016 Feb 1;22(3):657-69. doi: 10.1158/1078-0432.CCR-15-0234. Epub 2015 Sep 29. Clin Cancer Res. 2016. PMID: 26420854 Free PMC article.
-
Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes.Cancers (Basel). 2019 Jan 24;11(2):138. doi: 10.3390/cancers11020138. Cancers (Basel). 2019. PMID: 30682811 Free PMC article. Review.
-
Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy.Gynecol Oncol Res Pract. 2017 Jun 2;4:9. doi: 10.1186/s40661-017-0046-9. eCollection 2017. Gynecol Oncol Res Pract. 2017. PMID: 28588899 Free PMC article. Review.
Cited by
-
Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma-A Review.Vaccines (Basel). 2023 Mar 13;11(3):634. doi: 10.3390/vaccines11030634. Vaccines (Basel). 2023. PMID: 36992219 Free PMC article. Review.
-
Albumin and interferon-β fusion protein serves as an effective vaccine adjuvant to enhance antigen-specific CD8+ T cell-mediated antitumor immunity.J Immunother Cancer. 2022 Apr;10(4):e004342. doi: 10.1136/jitc-2021-004342. J Immunother Cancer. 2022. PMID: 35459734 Free PMC article.
-
Microneedle-Mediated Vaccine Delivery to the Oral Mucosa.Adv Healthc Mater. 2019 Feb;8(4):e1801180. doi: 10.1002/adhm.201801180. Epub 2018 Dec 10. Adv Healthc Mater. 2019. PMID: 30537400 Free PMC article. Review.
-
Head and Neck Squamous Cell Carcinoma: Risk Factors, Molecular Alterations, Immunology and Peptide Vaccines.Int J Pept Res Ther. 2022;28(1):19. doi: 10.1007/s10989-021-10334-5. Epub 2021 Dec 8. Int J Pept Res Ther. 2022. PMID: 34903958 Free PMC article. Review.
-
Optimal long peptide for flagellin-adjuvanted HPV E7 cancer vaccine to enhance tumor suppression in combination with anti-PD-1.Transl Cancer Res. 2022 Jun;11(6):1595-1602. doi: 10.21037/tcr-21-2798. Transl Cancer Res. 2022. PMID: 35836530 Free PMC article.
References
-
- Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

