Ontogeny of Tumor-associated Macrophages and Its Implication in Cancer Regulation
- PMID: 26949745
- PMCID: PMC4772875
- DOI: 10.1016/j.trecan.2015.11.004
Ontogeny of Tumor-associated Macrophages and Its Implication in Cancer Regulation
Abstract
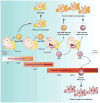
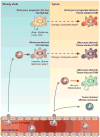
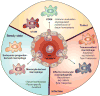
Macrophages are innate immune cells with evolutionarily conserved functions in tissue maintenance and host defense. As such, macrophages are among the first hematopoietic cells that seed developing tissues, and respond to inflammatory insults by in situ proliferation or de novo differentiation from monocytes. Recent studies have revealed that monocyte-derived tumor-induced macrophages represent a major tumor-associated macrophage population, which can further expand following their differentiation in tumors. Compared to tissue-resident tumor-associated macrophages, these newly differentiated cells are phenotypically distinct, and likely play a unique role in tissue dysregulation and immune modulation in cancer. These findings imply that tumor growth elicits a specific innate immune response. In this review, we explore the different routes of macrophage seeding and maintenance in tissues during steady state and inflammation and how these principles underlie the responses observed during tumor development. In addition, we highlight the relationship between the origin and function of macrophages in different settings and how this knowledge may be used to create new opportunities for cancer immunotherapy.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
