Polo-Like Kinase 3 Appears Dispensable for Normal Retinal Development Despite Robust Embryonic Expression
- PMID: 26949938
- PMCID: PMC4780821
- DOI: 10.1371/journal.pone.0150878
Polo-Like Kinase 3 Appears Dispensable for Normal Retinal Development Despite Robust Embryonic Expression
Abstract
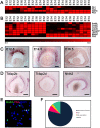
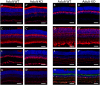
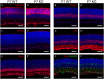
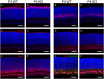
During retinogenesis seven different cell types are generated in distinct yet overlapping timepoints from a population of retinal progenitor cells. Previously, we performed single cell transcriptome analyses of retinal progenitor cells to identify candidate genes that may play roles in the generation of early-born retinal neurons. Based on its expression pattern in subsets of early retinal cells, polo-like kinase 3 (Plk3) was identified as one such candidate gene. Further characterization of Plk3 expression by in situ hybridization revealed that this gene is expressed as cells exit the cell cycle. We obtained a Plk3 deficient mouse and investigated changes in the retina's morphology and transcriptome through immunohistochemistry, in situ hybridization and gene expression profiling. These experiments have been performed initially on adult mice and subsequently extended throughout retinal development. Although morphological studies revealed no consistent changes in retinogenesis upon Plk3 loss, microarray profiling revealed potential candidate genes altered in Plk3-KO mice. Further studies will be necessary to understand the connection between these changes in gene expression and the loss of a protein kinase such as Plk3.
Conflict of interest statement
Figures



Similar articles
-
Cytoskeletal architecture and cell motility remain unperturbed in mouse embryonic fibroblasts from Plk3 knockout mice.Exp Biol Med (Maywood). 2016 Mar;241(6):603-10. doi: 10.1177/1535370216629010. Epub 2016 Feb 2. Exp Biol Med (Maywood). 2016. PMID: 26843517 Free PMC article.
-
Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors.Int J Oncol. 2002 Jan;20(1):121-6. Int J Oncol. 2002. PMID: 11743651
-
In vivo modulation of polo-like kinases supports a key role for PLK2 in Ser129 α-synuclein phosphorylation in mouse brain.Neuroscience. 2014 Jan 3;256:72-82. doi: 10.1016/j.neuroscience.2013.09.061. Epub 2013 Oct 12. Neuroscience. 2014. PMID: 24128992
-
Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues.Oncogene. 2005 Jan 10;24(2):260-6. doi: 10.1038/sj.onc.1208219. Oncogene. 2005. PMID: 15640841 Review.
-
Somatic gene targeting in the developing and adult mouse retina.Methods. 2002 Dec;28(4):457-64. doi: 10.1016/s1046-2023(02)00265-7. Methods. 2002. PMID: 12507464 Review.
Cited by
-
The Trim family of genes and the retina: Expression and functional characterization.PLoS One. 2018 Sep 12;13(9):e0202867. doi: 10.1371/journal.pone.0202867. eCollection 2018. PLoS One. 2018. PMID: 30208054 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

