Hypothalamic control of male aggression-seeking behavior
- PMID: 26950005
- PMCID: PMC4853470
- DOI: 10.1038/nn.4264
Hypothalamic control of male aggression-seeking behavior
Abstract
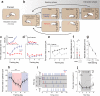
In many vertebrate species, certain individuals will seek out opportunities for aggression, even in the absence of threat-provoking cues. Although several brain areas have been implicated in the generation of attack in response to social threat, little is known about the neural mechanisms that promote self-initiated or 'voluntary' aggression-seeking when no threat is present. To explore this directly, we utilized an aggression-seeking task in which male mice self-initiated aggression trials to gain brief and repeated access to a weaker male that they could attack. In males that exhibited rapid task learning, we found that the ventrolateral part of the ventromedial hypothalamus (VMHvl), an area with a known role in attack, was essential for aggression-seeking. Using both single-unit electrophysiology and population optical recording, we found that VMHvl neurons became active during aggression-seeking and that their activity tracked changes in task learning and extinction. Inactivation of the VMHvl reduced aggression-seeking behavior, whereas optogenetic stimulation of the VMHvl accelerated moment-to-moment aggression-seeking and intensified future attack. These data demonstrate that the VMHvl can mediate both acute attack and flexible seeking actions that precede attack.
Figures





Comment in
-
Fight club for mice.Nat Neurosci. 2016 Apr;19(4):532. doi: 10.1038/nn0416-532. Nat Neurosci. 2016. PMID: 27021943 No abstract available.
References
-
- Fish EW, De Bold JF, Miczek KA. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology. 2002;163:459–466. doi:DOI 10.1007/s00213-002-1211-2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

