Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function
- PMID: 26950270
- PMCID: PMC5014730
- DOI: 10.1002/ana.24631
Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function
Abstract
Objective: The Epi4K Consortium recently identified 4 de novo mutations in the γ-aminobutyric acid type A (GABAA ) receptor β3 subunit gene GABRB3 and 1 in the β1 subunit gene GABRB1 in children with one of the epileptic encephalopathies (EEs) Lennox-Gastaut syndrome (LGS) and infantile spasms (IS). Because the etiology of EEs is often unknown, we determined the impact of GABRB mutations on GABAA receptor function and biogenesis.
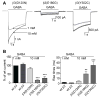
Methods: GABAA receptor α1 and γ2L subunits were coexpressed with wild-type and/or mutant β3 or β1 subunits in HEK 293T cells. Currents were measured using whole cell and single channel patch clamp techniques. Surface and total expression levels were measured using flow cytometry. Potential structural perturbations in mutant GABAA receptors were explored using structural modeling.
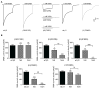
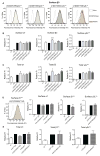
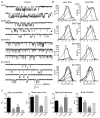
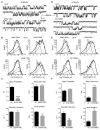
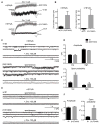
Results: LGS-associated GABRB3(D120N, E180G, Y302C) mutations located at β+ subunit interfaces reduced whole cell currents by decreasing single channel open probability without loss of surface receptors. In contrast, IS-associated GABRB3(N110D) and GABRB1(F246S) mutations at β- subunit interfaces produced minor changes in whole cell current peak amplitude but altered current deactivation by decreasing or increasing single channel burst duration, respectively. GABRB3(E180G) and GABRB1(F246S) mutations also produced spontaneous channel openings.
Interpretation: All 5 de novo GABRB mutations impaired GABAA receptor function by rearranging conserved structural domains, supporting their role in EEs. The primary effect of LGS-associated mutations was reduced GABA-evoked peak current amplitudes, whereas the major impact of IS-associated mutations was on current kinetic properties. Despite lack of association with epilepsy syndromes, our results suggest GABRB1 as a candidate human epilepsy gene. Ann Neurol 2016;79:806-825.
© 2016 American Neurological Association.
Conflict of interest statement
Figures



Comment in
-
Reply.Ann Neurol. 2016 Aug;80(2):312-3. doi: 10.1002/ana.24701. Epub 2016 Jun 24. Ann Neurol. 2016. PMID: 27270493 No abstract available.
-
A second patient with a De Novo GABRB1 mutation and epileptic encephalopathy.Ann Neurol. 2016 Aug;80(2):311-2. doi: 10.1002/ana.24699. Epub 2016 Jun 28. Ann Neurol. 2016. PMID: 27273810 No abstract available.
References
-
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010 Apr;51(4):676–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

