USP7 is a SUMO deubiquitinase essential for DNA replication
- PMID: 26950370
- PMCID: PMC4869841
- DOI: 10.1038/nsmb.3185
USP7 is a SUMO deubiquitinase essential for DNA replication
Abstract
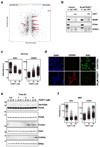
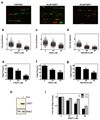
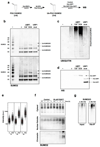
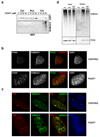
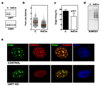
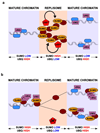
Post-translational modification of proteins by ubiquitin (Ub) and Ub-like modifiers regulates DNA replication. We have previously shown that chromatin around replisomes is rich in SUMO and poor in Ub, whereas mature chromatin exhibits an opposite pattern. How this SUMO-rich, Ub-poor environment is maintained at sites of DNA replication in mammalian cells remains unexplored. Here we identify USP7 as a replisome-enriched SUMO deubiquitinase that is essential for DNA replication. By acting on SUMO and SUMOylated proteins, USP7 counteracts their ubiquitination. Inhibition or genetic deletion of USP7 leads to the accumulation of Ub on SUMOylated proteins, which are displaced away from replisomes. Our findings provide a model explaining the differential accumulation of SUMO and Ub at replication forks and identify an essential role of USP7 in DNA replication that should be considered in the development of USP7 inhibitors as anticancer agents.
Figures

References
-
- Lehmann AR. Ubiquitin-family modifications in the replication of DNA damage. FEBS Lett. 2011;585:2772–9. - PubMed
-
- Zhang W, Qin Z, Zhang X, Xiao W. Roles of sequential ubiquitination of PCNA in DNA-damage tolerance. FEBS Lett. 2011;585:2786–94. - PubMed
-
- Huang TT, et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006;8:339–47. - PubMed
-
- Cohn MA, et al. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell. 2007;28:786–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

