Decrease of VEGF-A in myeloid cells attenuates glioma progression and prolongs survival in an experimental glioma model
- PMID: 26951383
- PMCID: PMC4896547
- DOI: 10.1093/neuonc/now005
Decrease of VEGF-A in myeloid cells attenuates glioma progression and prolongs survival in an experimental glioma model
Abstract
Background: Glioblastomas are highly vascularized tumors with a prominent infiltration of macrophages/microglia whose role in promoting glioma growth, invasion, and angiogenesis has not been fully elucidated.
Methods: The contribution of myeloid-derived vascular endothelial growth factor (VEGF) to glioma growth was analyzed in vivo in a syngeneic intracranial GL261 glioma model using a Cre/loxP system to knock out the expression of VEGF-A in CD11b + myeloid cells. Changes in angiogenesis-related gene expression profile were analyzed in mutant bone marrow-derived (BMD) macrophages in vitro. Furthermore, we studied the influence of macrophages on GL261 growth, invasiveness, and protein expression profile of angiogenic molecules as well as the paracrine effect of mutant macrophages on angiogenesis in vitro.
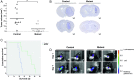
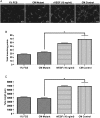
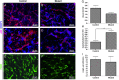
Results: Myeloid cell-restricted VEGF-A deficiency leads to a growth delay of intracranial tumors and prolonged survival. The tumor vasculature in mutant mice was more regular, with increased pericyte coverage. Expression analysis revealed significant downregulation of VEGF-A and slight upregulation of TGFβ-1 in BMD macrophages from mutant mice. Endothelial tube formation was significantly decreased by conditioned media from mutant macrophages. The expression of angiogenesis-related proteins in GL261 glioma cells in co-culture experiments either with wild-type or mutant macrophages remained unchanged, indicating that effects observed in vivo are due to myeloid-derived VEGF-A deficiency.
Conclusions: Our results highlight the importance of VEGF derived from tumor-infiltrating myeloid cells for initiating vascularization in gliomas. The combination of antiangiogenic agents with myeloid cell-targeting strategies might provide a new therapeutic approach for glioblastoma patients.
Keywords: VEGF; angiogenesis; glioma; macrophages; microglia.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Machein M, de Miguel LS. Angiogenesis in gliomas. Recent Results Cancer Res. 2009;171:193–215. - PubMed
-
- Machein MR, Plate KH. VEGF in brain tumors. J Neurooncol. 2000;50(1–2):109–120. - PubMed
-
- Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41(9):2522–2525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

