Computer vision cracks the leaf code
- PMID: 26951664
- PMCID: PMC4812720
- DOI: 10.1073/pnas.1524473113
Computer vision cracks the leaf code
Abstract
Understanding the extremely variable, complex shape and venation characters of angiosperm leaves is one of the most challenging problems in botany. Machine learning offers opportunities to analyze large numbers of specimens, to discover novel leaf features of angiosperm clades that may have phylogenetic significance, and to use those characters to classify unknowns. Previous computer vision approaches have primarily focused on leaf identification at the species level. It remains an open question whether learning and classification are possible among major evolutionary groups such as families and orders, which usually contain hundreds to thousands of species each and exhibit many times the foliar variation of individual species. Here, we tested whether a computer vision algorithm could use a database of 7,597 leaf images from 2,001 genera to learn features of botanical families and orders, then classify novel images. The images are of cleared leaves, specimens that are chemically bleached, then stained to reveal venation. Machine learning was used to learn a codebook of visual elements representing leaf shape and venation patterns. The resulting automated system learned to classify images into families and orders with a success rate many times greater than chance. Of direct botanical interest, the responses of diagnostic features can be visualized on leaf images as heat maps, which are likely to prompt recognition and evolutionary interpretation of a wealth of novel morphological characters. With assistance from computer vision, leaves are poised to make numerous new contributions to systematic and paleobotanical studies.
Keywords: computer vision; leaf architecture; leaf venation; paleobotany; sparse coding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



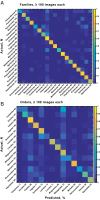
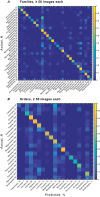
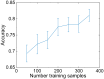
References
-
- Hickey LJ, Wolfe JA. The bases of angiosperm phylogeny: Vegetative morphology. Ann Mo Bot Gard. 1975;62(3):538–589.
-
- Hickey LJ, Doyle JA. Early Cretaceous fossil evidence for angiosperm evolution. Bot Rev. 1977;43(1):2–104.
-
- Doyle JA. Systematic value and evolution of leaf architecture across the angiosperms in light of molecular phylogenetic analyses. Cour Forschungsinst Senckenb. 2007;258:21–37.
-
- Soltis DE, Soltis PS, Endress PK, Chase MW. Phylogeny and Evolution of Angiosperms. Sinauer; Sunderland, MA: 2005.
-
- Friis EM, Crane PR, Pedersen KR. Early Flowers and Angiosperm Evolution. Cambridge Univ Press; Cambridge, UK: 2011.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

