Study protocol of SWEPIS a Swedish multicentre register based randomised controlled trial to compare induction of labour at 41 completed gestational weeks versus expectant management and induction at 42 completed gestational weeks
- PMID: 26951777
- PMCID: PMC4782290
- DOI: 10.1186/s12884-016-0836-9
Study protocol of SWEPIS a Swedish multicentre register based randomised controlled trial to compare induction of labour at 41 completed gestational weeks versus expectant management and induction at 42 completed gestational weeks
Abstract
Background: Observational data shows that postterm pregnancy (≥42 gestational weeks, GW) and late term pregnancy (≥41 GW), as compared to term pregnancy, is associated with an increased risk for adverse outcome for the mother and infant. Standard care in many countries is induction of labour at 42 GW. There is insufficient scientific support that induction of labour at 41 GW, as compared with expectant management and induction at 42 GW will reduce perinatal mortality and morbidity without an increase in operative deliveries, negative delivery experiences or higher costs. Large randomised studies are needed since important outcomes; such as perinatal mortality and hypoxic ischaemic encephalopathy are rare events.
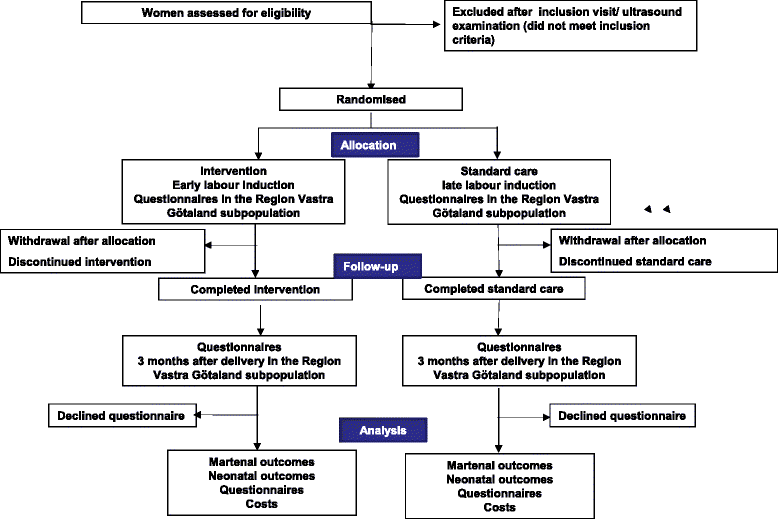
Methods/design: A total of 10 038 healthy women ≥18 years old with a normal live singleton pregnancy in cephalic presentation at 41 GW estimated with a first or second trimester ultrasound, who is able to understand oral and written information will be randomised to labour induction at 41 GW (early induction) or expectant management and induction at 42 GW (late induction). Women will be recruited at university clinics and county hospitals in Sweden comprising more than 65 000 deliveries per year. Primary outcome will be a composite of stillbirth, neonatal mortality and severe neonatal morbidity. Secondary outcomes will be other adverse neonatal and maternal outcomes, mode of delivery, women's experience, cost effectiveness and infant morbidity up to 3 months of age. Data on background variables, obstetric and neonatal outcomes will be obtained from the Swedish Pregnancy Register and the Swedish Neonatal Quality Register. Data on women's experiences will be collected by questionnaires after randomisation and 3 months after delivery. Primary analysis will be intention to treat. The statistician will be blinded to group and intervention.
Discussion: It is important to investigate if an intervention at 41 GW is superior to standard care in order to reduce death and lifelong disability for the children. The pregnant population, >41 GW, constitutes 15-20% of all pregnancies and the results of the study will thus have a great impact. The use of registries for randomisation and collection of outcome data represents a unique and new study design.
Trial registration: The study was registered in Current Controlled Trials, ISRCTN26113652 the 30(th) of March 2015 (DOI 10.1186/ISRCTN26113652 ).
References
-
- WHO Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977;56:247–253. - PubMed
-
- Pregnancies, Deliveries and Newborn Infants . The Swedish Medical Birth Register 1973–2013. Stockholm: Socialstyrelsen; 2014.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical