Use of transient elastography in patients with HIV-HCV coinfection: A systematic review and meta-analysis
- PMID: 26952020
- PMCID: PMC5014713
- DOI: 10.1111/jgh.13337
Use of transient elastography in patients with HIV-HCV coinfection: A systematic review and meta-analysis
Abstract
Background and aim: Patients with HIV-hepatitis C virus (HCV) coinfection progress towards liver fibrosis and cirrhosis more rapidly compared with HCV mono-infected individuals. This necessitates an accurate assessment of liver stiffness with transient elastography to guide treatment.
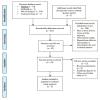
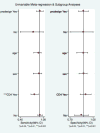
Methods: Searches of PubMed, EMBASE, Web of Science, and the Cochrane Library databases were performed through January 2016 to assess the diagnostic accuracy of transient elastography for liver stiffness in the HIV-HCV population. Included studies were analyzed according to the Cochrane DTA Working Group methodology. Bivariate and hierarchical models were used to compute pooled sensitivity and specificity. Positive and negative likelihood ratios were also determined. A Fagan nomogram was constructed. Meta-regression analysis was performed with assessment of publication bias using Deeks' funnel plot asymmetry testing.
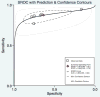
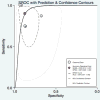
Results: A total of six studies (n = 756) met the inclusion criteria. The diagnostic accuracy of elastography for the diagnosis of moderate (≥F2) fibrosis was 88% (95% confidence interval [CI], 0.85-0.90). The pooled sensitivity and specificity of moderate fibrosis was 97% (95% CI, 0.82-0.91) and 64% (95% CI, 0.45-0.79), respectively. The diagnostic accuracy of elastography for the assessment of cirrhosis was 94% (95% CI, 0.91-0.95). The pooled sensitivity and specificity for cirrhosis was 90% (95% CI, 0.74-0.97) and 87% (95% CI, 0.80-0.92), respectively. Meta-regression analysis demonstrated that CD4 cell count did not impact diagnostic accuracy of elastography.
Conclusions: Transient elastography is a noninvasive imaging modality with excellent ability to assess for cirrhosis in patients with HIV-HCV coinfection.
Keywords: HCV; HIV; elastography; fibrosis; hepatitis.
© 2016 Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Potential Conflicts of Interest: The authors have no potential conflicts of interest to report.
Figures




References
-
- European Association for Study of L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of hepatology. 2014;60:392–420. - PubMed
-
- Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–51. - PubMed
-
- Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. - PubMed
-
- HIV/AIDS and Viral Hepatitis. Viral Hepatitis - CDC Recommendations for Specific Populations and Settings. [Accessed 16 January 2016]; http://www.cdc.gov/hepatitis/Populations/HIV.htm.
-
- Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Seminars in liver disease. 2012;32:103–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

