Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial
- PMID: 26952094
- PMCID: PMC4782330
- DOI: 10.1186/s12916-016-0581-y
Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial
Abstract
Background: A single low dose (0.25 mg/kg) of primaquine is recommended as a gametocytocide in combination with artemisinin-based combination therapies for Plasmodium falciparum but its effect on post-treatment gametocyte circulation and infectiousness to mosquitoes has not been quantified.
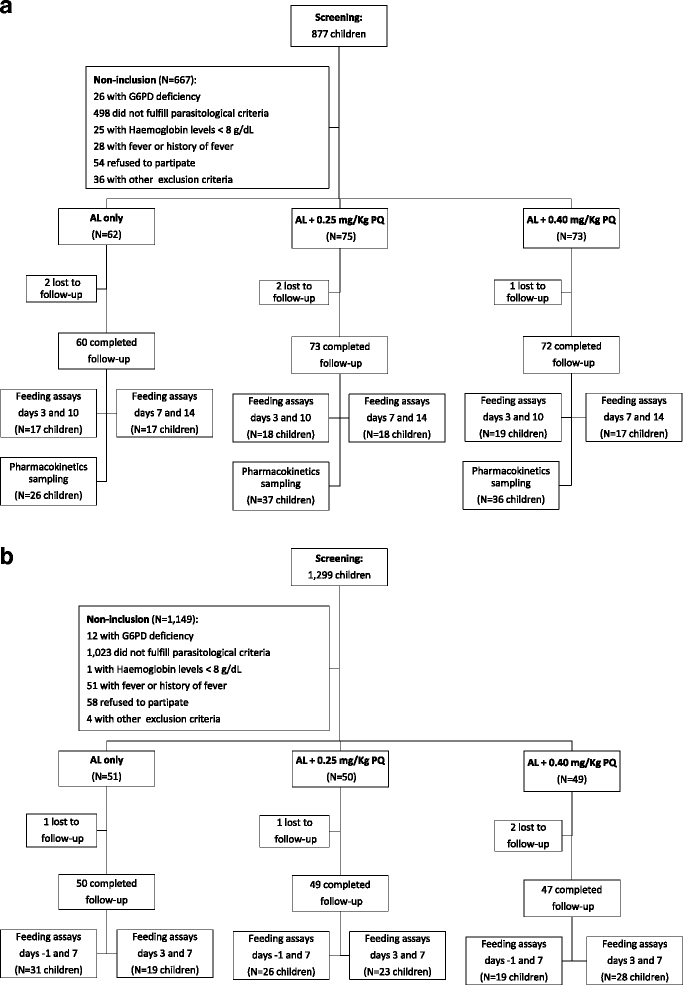
Methods: In this randomised, double-blind, placebo-controlled trial, 360 asymptomatic parasitaemic children aged 2-15 years were enrolled and assigned to receive: artemether-lumefantrine (AL) and a dose of placebo; AL and a 0.25 mg/kg primaquine dose; or AL and a 0.40 mg/kg primaquine dose. On days 0, 2, 3, 7, 10 and 14, gametocytes were detected and quantified by microscopy, Pfs25 mRNA quantitative nucleic acid sequence based amplification (QT-NASBA), and quantitative reverse-transcriptase PCR (qRT-PCR). For a subset of participants, pre- and post-treatment infectiousness was assessed by mosquito feeding assays on days -1, 3, 7, 10 and 14.
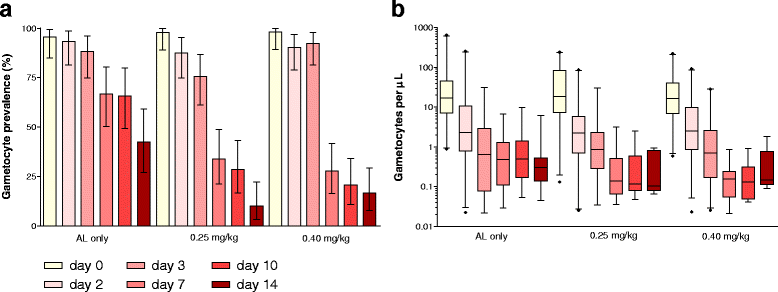
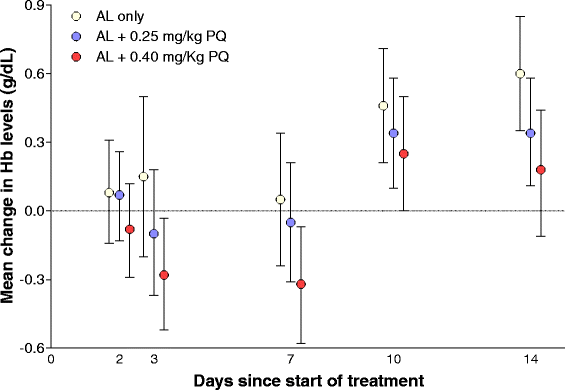
Results: Both primaquine arms had lower gametocyte prevalences after day 3 compared to the placebo arm, regardless of gametocyte detection method. The mean (95% confidence interval) number of days to gametocyte clearance in children with patent gametocytes on day 0 (N = 150) was 19.7 (14.6 - 24.8), 7.7 (6.3 - 9.1) and 8.2 (6.7 - 9.6) for the AL-placebo, the 0.25 mg/kg primaquine dose and the 0.40 mg/kg primaquine dose arms, respectively. While 38.0% (30/79) of selected gametocytaemic individuals were infectious before treatment, only 1/251 participant, from the AL-placebo group, infected mosquitoes after treatment.
Conclusions: We observed similar gametocyte clearance rates after 0.25 and 0.40 mg/kg primaquine doses. Infectivity to mosquitoes after AL was very low and absent in primaquine arms. CLINICALTRIALS.
Gov registration: NCT01935882.
Figures
References
-
- WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2010. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

