Trifunctional cross-linker for mapping protein-protein interaction networks and comparing protein conformational states
- PMID: 26952210
- PMCID: PMC4811778
- DOI: 10.7554/eLife.12509
Trifunctional cross-linker for mapping protein-protein interaction networks and comparing protein conformational states
Abstract
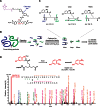
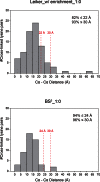
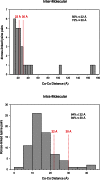
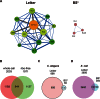
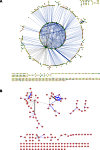
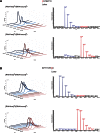
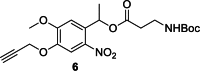
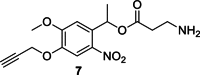
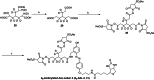
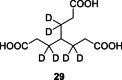
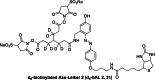
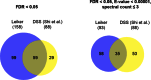
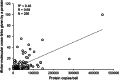
To improve chemical cross-linking of proteins coupled with mass spectrometry (CXMS), we developed a lysine-targeted enrichable cross-linker containing a biotin tag for affinity purification, a chemical cleavage site to separate cross-linked peptides away from biotin after enrichment, and a spacer arm that can be labeled with stable isotopes for quantitation. By locating the flexible proteins on the surface of 70S ribosome, we show that this trifunctional cross-linker is effective at attaining structural information not easily attainable by crystallography and electron microscopy. From a crude Rrp46 immunoprecipitate, it helped identify two direct binding partners of Rrp46 and 15 protein-protein interactions (PPIs) among the co-immunoprecipitated exosome subunits. Applying it to E. coli and C. elegans lysates, we identified 3130 and 893 inter-linked lysine pairs, representing 677 and 121 PPIs. Using a quantitative CXMS workflow we demonstrate that it can reveal changes in the reactivity of lysine residues due to protein-nucleic acid interaction.
Keywords: 70S ribosome; c. elegans; e. coli; biophysics; cross-linking; exosome; mass spectrometry; protein structure; protein-protein interactions; structural biology.
Conflict of interest statement
The author declares that no competing interests exist.
Figures





















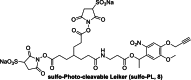






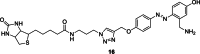





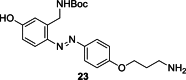

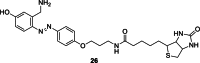






























References
-
- Bohn S, Beck F, Sakata E, Walzthoeni T, Beck M, Aebersold R, Förster F, Baumeister W, Nickell S. Structure of the 26S proteasome from Schizosaccharomyces pombe at subnanometer resolution. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20992–20997. doi: 10.1073/pnas.1015530107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

