A ligand-independent integrin β1 mechanosensory complex guides spindle orientation
- PMID: 26952307
- PMCID: PMC4786777
- DOI: 10.1038/ncomms10899
A ligand-independent integrin β1 mechanosensory complex guides spindle orientation
Abstract
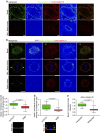
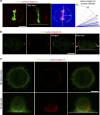
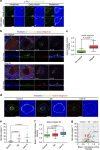
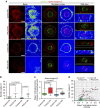
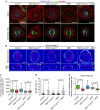
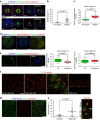
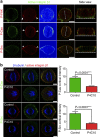
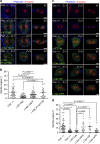
Control of spindle orientation is a fundamental process for embryonic development, morphogenesis and tissue homeostasis, while defects are associated with tumorigenesis and other diseases. Force sensing is one of the mechanisms through which division orientation is determined. Here we show that integrin β1 plays a critical role in this process, becoming activated at the lateral regions of the cell cortex in a ligand-independent manner. This activation is force dependent and polar, correlating with the spindle capture sites. Inhibition of integrin β1 activation on the cortex and disruption of its asymmetric distribution leads to spindle misorientation, even when cell adhesion is β1 independent. Examining downstream targets reveals that a cortical mechanosensory complex forms on active β1, and regulates spindle orientation irrespective of cell context. We propose that ligand-independent integrin β1 activation is a conserved mechanism that allows cell responses to external stimuli.
Figures

Similar articles
-
PCTK1 regulates integrin-dependent spindle orientation via protein kinase A regulatory subunit KAP0 and myosin X.Mol Cell Biol. 2015 Apr;35(7):1197-208. doi: 10.1128/MCB.01017-14. Epub 2015 Jan 20. Mol Cell Biol. 2015. PMID: 25605337 Free PMC article.
-
Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner.EMBO J. 2007 Mar 21;26(6):1487-98. doi: 10.1038/sj.emboj.7601599. Epub 2007 Feb 22. EMBO J. 2007. PMID: 17318179 Free PMC article.
-
Interphase adhesion geometry is transmitted to an internal regulator for spindle orientation via caveolin-1.Nat Commun. 2016 Jun 13;7:ncomms11858. doi: 10.1038/ncomms11858. Nat Commun. 2016. PMID: 27292265 Free PMC article.
-
Spindle orientation in animal cell mitosis: roles of integrin in the control of spindle axis.J Cell Physiol. 2007 Nov;213(2):407-11. doi: 10.1002/jcp.21227. J Cell Physiol. 2007. PMID: 17654475 Review.
-
Orientation of the Mitotic Spindle in the Development of Tubular Organs.J Cell Biochem. 2017 Jul;118(7):1630-1633. doi: 10.1002/jcb.25865. Epub 2017 Mar 21. J Cell Biochem. 2017. PMID: 28059469 Review.
Cited by
-
Epigenetic regulation of asymmetric cell division by the LIBR-BRD4 axis.Nucleic Acids Res. 2024 Jan 11;52(1):154-165. doi: 10.1093/nar/gkad1095. Nucleic Acids Res. 2024. PMID: 37986225 Free PMC article.
-
Multiscale force sensing in development.Nat Cell Biol. 2017 May 31;19(6):581-588. doi: 10.1038/ncb3524. Nat Cell Biol. 2017. PMID: 28561050 Review.
-
The balance between adhesion and contraction during cell division.Curr Opin Cell Biol. 2019 Feb;56:45-52. doi: 10.1016/j.ceb.2018.09.001. Epub 2018 Sep 28. Curr Opin Cell Biol. 2019. PMID: 30268802 Free PMC article. Review.
-
Mechanical stretching stimulates growth of the basal layer and rete ridges in the epidermis.J Tissue Eng Regen Med. 2019 Nov;13(11):2121-2125. doi: 10.1002/term.2952. Epub 2019 Sep 5. J Tissue Eng Regen Med. 2019. PMID: 31381259 Free PMC article.
-
β1 integrin is a sensor of blood flow direction.J Cell Sci. 2019 Jun 3;132(11):jcs229542. doi: 10.1242/jcs.229542. J Cell Sci. 2019. PMID: 31076511 Free PMC article.
References
-
- Morin X. & Bellaiche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell 21, 102–119 (2011). - PubMed
-
- Pease J. C. & Tirnauer J. S. Mitotic spindle misorientation in cancer--out of alignment and into the fire. J. Cell Sci. 124, 1007–1016 (2011). - PubMed
-
- Fink J. et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 13, 771–778 (2011). - PubMed
-
- Petridou N. I. & Skourides P. A. FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat. Commun. 5, 5240 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

