Glutathione-deficient Plasmodium berghei parasites exhibit growth delay and nuclear DNA damage
- PMID: 26952808
- PMCID: PMC4934901
- DOI: 10.1016/j.freeradbiomed.2016.02.032
Glutathione-deficient Plasmodium berghei parasites exhibit growth delay and nuclear DNA damage
Abstract
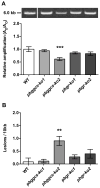
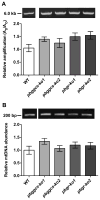
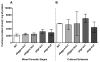
Plasmodium parasites are exposed to endogenous and exogenous oxidative stress during their complex life cycle. To minimize oxidative damage, the parasites use glutathione (GSH) and thioredoxin (Trx) as primary antioxidants. We previously showed that disruption of the Plasmodium berghei gamma-glutamylcysteine synthetase (pbggcs-ko) or the glutathione reductase (pbgr-ko) genes resulted in a significant reduction of GSH in intraerythrocytic stages, and a defect in growth in the pbggcs-ko parasites. In this report, time course experiments of parasite intraerythrocytic development and morphological studies showed a growth delay during the ring to schizont progression. Morphological analysis shows a significant reduction in size (diameter) of trophozoites and schizonts with increased number of cytoplasmic vacuoles in the pbggcs-ko parasites in comparison to the wild type (WT). Furthermore, the pbggcs-ko mutants exhibited an impaired response to oxidative stress and increased levels of nuclear DNA (nDNA) damage. Reduced GSH levels did not result in mitochondrial DNA (mtDNA) damage or protein carbonylations in neither pbggcs-ko nor pbgr-ko parasites. In addition, the pbggcs-ko mutant parasites showed an increase in mRNA expression of genes involved in oxidative stress detoxification and DNA synthesis, suggesting a potential compensatory mechanism to allow for parasite proliferation. These results reveal that low GSH levels affect parasite development through the impairment of oxidative stress reduction systems and damage to the nDNA. Our studies provide new insights into the role of the GSH antioxidant system in the intraerythrocytic development of Plasmodium parasites, with potential translation into novel pharmacological interventions.
Keywords: DNA damage; Glutathione; Growth delay; Malaria; Oxidative stress; Plasmodium berghei; Protein carbonylations.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no competing interests to declare.
Figures


Similar articles
-
Implications of Glutathione Levels in the Plasmodium berghei Response to Chloroquine and Artemisinin.PLoS One. 2015 May 26;10(5):e0128212. doi: 10.1371/journal.pone.0128212. eCollection 2015. PLoS One. 2015. PMID: 26010448 Free PMC article.
-
The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission.PLoS Pathog. 2009 Feb;5(2):e1000302. doi: 10.1371/journal.ppat.1000302. Epub 2009 Feb 20. PLoS Pathog. 2009. PMID: 19229315 Free PMC article.
-
Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito.J Biol Chem. 2010 Aug 27;285(35):27045-27056. doi: 10.1074/jbc.M110.122275. Epub 2010 Jun 23. J Biol Chem. 2010. PMID: 20573956 Free PMC article.
-
Thioredoxin reductase and glutathione synthesis in Plasmodium falciparum.Redox Rep. 2003;8(5):251-5. doi: 10.1179/135100003225002853. Redox Rep. 2003. PMID: 14962359 Review.
-
Redox and antioxidant systems of the malaria parasite Plasmodium falciparum.Mol Microbiol. 2004 Sep;53(5):1291-305. doi: 10.1111/j.1365-2958.2004.04257.x. Mol Microbiol. 2004. PMID: 15387810 Review.
Cited by
-
Cellular mechanisms of action and resistance of Plasmodium falciparum to artemisinin.Parasitol Res. 2017 Dec;116(12):3331-3339. doi: 10.1007/s00436-017-5647-z. Epub 2017 Nov 10. Parasitol Res. 2017. PMID: 29127525
-
Genome-Wide CRISPR/Cas9 Screen Identifies New Genes Critical for Defense Against Oxidant Stress in Toxoplasma gondii.Front Microbiol. 2021 Jun 7;12:670705. doi: 10.3389/fmicb.2021.670705. eCollection 2021. Front Microbiol. 2021. PMID: 34163449 Free PMC article.
-
Formononetin exerts synergistic action with artesunate against multi-drug-resistant P. falciparum arresting ring-to-schizont transition by inducing reactive oxygen species.Arch Microbiol. 2025 Apr 22;207(6):128. doi: 10.1007/s00203-025-04321-3. Arch Microbiol. 2025. PMID: 40261423
-
Beta vulgaris Betalains Mitigate Parasitemia and Brain Oxidative Stress Induced by Plasmodium berghei in Mice.Pharmaceuticals (Basel). 2024 Aug 13;17(8):1064. doi: 10.3390/ph17081064. Pharmaceuticals (Basel). 2024. PMID: 39204168 Free PMC article.
-
Tafenoquine Is a Promising Drug Candidate for the Treatment of Babesiosis.Antimicrob Agents Chemother. 2021 Jun 17;65(7):e0020421. doi: 10.1128/AAC.00204-21. Epub 2021 Jun 17. Antimicrob Agents Chemother. 2021. PMID: 33941516 Free PMC article.
References
-
- World Health Organization (WHO) [accessed 11.19.14];World Malaria Report 2014. 2014 http://www.who.int/malaria/publications/world_malaria_report_2014/en/ind....
-
- Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol. 2004;34:163–189. - PubMed
-
- Müller S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol Microbiol. 2004;53(5):1291–305. - PubMed
-
- Tilley L, Loria P, Foley M, editors. Antimalarial Chemotherapy. Vol. 2001. Humana Press; 2001. Chloroquine and other quinoline antimalarials; pp. 87–121.
-
- Krauth-Siegel RL, Bauer H, Schirmer RH. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew Chem Int Ed Engl. 2005;44:690–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

