Interplay between cardiac function and heart development
- PMID: 26952935
- PMCID: PMC4906158
- DOI: 10.1016/j.bbamcr.2016.03.004
Interplay between cardiac function and heart development
Abstract
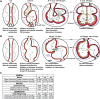
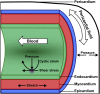
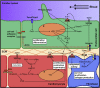
Mechanotransduction refers to the conversion of mechanical forces into biochemical or electrical signals that initiate structural and functional remodeling in cells and tissues. The heart is a kinetic organ whose form changes considerably during development and disease. This requires cardiomyocytes to be mechanically durable and able to mount coordinated responses to a variety of environmental signals on different time scales, including cardiac pressure loading and electrical and hemodynamic forces. During physiological growth, myocytes, endocardial and epicardial cells have to adaptively remodel to these mechanical forces. Here we review some of the recent advances in the understanding of how mechanical forces influence cardiac development, with a focus on fluid flow forces. This article is part of a Special Issue entitled: Cardiomyocyte Biology: Integration of Developmental and Environmental Cues in the Heart edited by Marcus Schaub and Hughes Abriel.
Keywords: Blood and pericardial flow; Cardiac development; Mechanosensing; Mechanotransduction; Mouse; Zebrafish.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

