Novel pharmacotherapies in diabetic retinopathy: Current status and what's in the horizon?
- PMID: 26953018
- PMCID: PMC4821120
- DOI: 10.4103/0301-4738.178154
Novel pharmacotherapies in diabetic retinopathy: Current status and what's in the horizon?
Abstract
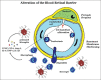
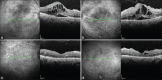
The blood-retinal barrier (BRB) alteration is the hallmark feature of diabetic retinopathy. Vascular endothelial growth factor (VEGF) is a potent vasopermeability factor that has been implicated in the pathogenesis of BRB alteration. Inflammation also plays a crucial role in this process with involvement of several chemokines and cytokines. Multiple anti-VEGF drugs are widely used as in the treatment of diabetic macular edema (DME) as well as proliferative diabetic retinopathy. Several clinical trials have proved the beneficial effects of these drugs in improvement of vision and prevention of vision loss. However, the response to anti-VEGF drugs in DME is not complete in a significant number of patients. The effect seems transient in this latter group, and many patients do not show complete resolution of fluid. Potential novel therapies targeting molecules beyond VEGF are being developed and examined in clinical trials.
Figures

Similar articles
-
Novel therapeutic targets in diabetic macular edema: Beyond VEGF.Vision Res. 2017 Oct;139:221-227. doi: 10.1016/j.visres.2017.06.015. Epub 2017 Oct 16. Vision Res. 2017. PMID: 28993218 Review.
-
Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets.Ophthalmology. 2015 Jul;122(7):1375-94. doi: 10.1016/j.ophtha.2015.03.024. Epub 2015 Apr 30. Ophthalmology. 2015. PMID: 25935789 Review.
-
[Pathophysiology and therapeutic progress of diabetic macular edema].Zhonghua Yan Ke Za Zhi. 2018 Aug 11;54(8):625-630. doi: 10.3760/cma.j.issn.0412-4081.2018.08.014. Zhonghua Yan Ke Za Zhi. 2018. PMID: 30107656 Review. Chinese.
-
Diabetic macular edema.Ophthalmologica. 2012;227 Suppl 1:21-9. doi: 10.1159/000337156. Epub 2012 Apr 24. Ophthalmologica. 2012. PMID: 22517122 Review.
-
Evidence for anti-VEGF treatment of diabetic macular edema.Ophthalmic Res. 2012;48 Suppl 1:16-20. doi: 10.1159/000339843. Epub 2012 Aug 21. Ophthalmic Res. 2012. PMID: 22907145 Review.
Cited by
-
Diabetic retinopathy: Battling the global epidemic.Indian J Ophthalmol. 2016 Jan;64(1):2-3. doi: 10.4103/0301-4738.178155. Indian J Ophthalmol. 2016. PMID: 26953017 Free PMC article. No abstract available.
-
Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography.J Diabetes Res. 2019 Dec 20;2019:8164250. doi: 10.1155/2019/8164250. eCollection 2019. J Diabetes Res. 2019. PMID: 31930145 Free PMC article. Review.
-
The role of inflammation in diabetic eye disease.Semin Immunopathol. 2019 Jul;41(4):427-445. doi: 10.1007/s00281-019-00750-7. Epub 2019 Jun 7. Semin Immunopathol. 2019. PMID: 31175392 Review.
-
Early response of anti-vascular endothelial growth factor (anti-VEGF) in diabetic macular edema (DME) management: microperimetry and optical coherence tomography (OCT) findings: a pilot study at national eye center of third world country.BMC Ophthalmol. 2024 Dec 27;24(1):551. doi: 10.1186/s12886-024-03744-8. BMC Ophthalmol. 2024. PMID: 39731082 Free PMC article.
-
Adjuvant Therapies in Diabetic Retinopathy as an Early Approach to Delay Its Progression: The Importance of Oxidative Stress and Inflammation.Oxid Med Cell Longev. 2020 Mar 11;2020:3096470. doi: 10.1155/2020/3096470. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32256949 Free PMC article. Review.
References
-
- Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–83. - PubMed
-
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. - PubMed
-
- Kuwabara T, Cogan DG. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963;69:492–502. - PubMed
-
- Das A, Frank RN, Weber ML, Kennedy A, Reidy CA, Mancini MA. ATP causes retinal pericytes to contract in vitro. Exp Eye Res. 1988;46:349–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

