Inhibition of DYRK1A Stimulates Human β-Cell Proliferation
- PMID: 26953159
- PMCID: PMC4878416
- DOI: 10.2337/db15-1127
Inhibition of DYRK1A Stimulates Human β-Cell Proliferation
Abstract
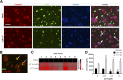
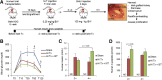
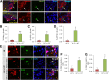
Restoring functional β-cell mass is an important therapeutic goal for both type 1 and type 2 diabetes (1). While proliferation of existing β-cells is the primary means of β-cell replacement in rodents (2), it is unclear whether a similar principle applies to humans, as human β-cells are remarkably resistant to stimulation of division (3,4). Here, we show that 5-iodotubercidin (5-IT), an annotated adenosine kinase inhibitor previously reported to increase proliferation in rodent and porcine islets (5), strongly and selectively increases human β-cell proliferation in vitro and in vivo. Remarkably, 5-IT also increased glucose-dependent insulin secretion after prolonged treatment. Kinome profiling revealed 5-IT to be a potent and selective inhibitor of the dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) and cell division cycle-like kinase families. Induction of β-cell proliferation by either 5-IT or harmine, another natural product DYRK1A inhibitor, was suppressed by coincubation with the calcineurin inhibitor FK506, suggesting involvement of DYRK1A and nuclear factor of activated T cells signaling. Gene expression profiling in whole islets treated with 5-IT revealed induction of proliferation- and cell cycle-related genes, suggesting that true proliferation is induced by 5-IT. Furthermore, 5-IT promotes β-cell proliferation in human islets grafted under the kidney capsule of NOD-scid IL2Rg(null) mice. These results point to inhibition of DYRK1A as a therapeutic strategy to increase human β-cell proliferation.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures





Comment in
-
DYRK1A: A Promising Drug Target for Islet Transplant-Based Diabetes Therapies.Diabetes. 2016 Jun;65(6):1496-8. doi: 10.2337/dbi16-0013. Diabetes. 2016. PMID: 27222397 No abstract available.
References
-
- Vetere A, Choudhary A, Burns SM, Wagner BK. Targeting the pancreatic β-cell to treat diabetes. Nat Rev Drug Discov 2014;13:278–289 - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

