Population Pharmacokinetics of Isavuconazole in Subjects with Mild or Moderate Hepatic Impairment
- PMID: 26953193
- PMCID: PMC4862513
- DOI: 10.1128/AAC.02942-15
Population Pharmacokinetics of Isavuconazole in Subjects with Mild or Moderate Hepatic Impairment
Abstract
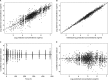
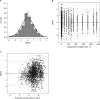
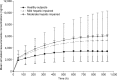
Isavuconazole, administered as the prodrug isavuconazonium sulfate, was recently approved by the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of adults with invasive aspergillosis and mucormycosis. The objective of this analysis was to develop a population pharmacokinetic model using NONMEM (version 7.2) for subjects with hepatic impairment, using intravenous and oral administration data from two hepatic studies, and to simulate concentration profiles to steady state, thus evaluating the need for dose adjustment. A two-compartment model with Weibull absorption function and first-order elimination process adequately described plasma isavuconazole concentrations. The population mean clearance in healthy subjects was 2.5 liters/h (5th and 95th percentiles: 2.0 and 3.1). The mean clearance values for subjects with mild and moderate hepatic impairment decreased approximately to 1.55 liters/h (5th and 95th percentiles: 1.3 and 1.8 liters/h) and 1.32 liters/h (5th and 95th percentiles: 1.05 and 1.35), respectively. Peripheral volume of distribution increased with body mass index. Simulations of mean concentration time profiles to steady state showed less than a 2-fold increase in mean trough concentrations for subjects with mild and moderate hepatic impairment compared with healthy subjects. After administration of the single dose, safety data for subjects with mild and moderate hepatic impairment were generally comparable to those for healthy subjects in both studies. Due to the <2-fold increase in trough concentrations and the established safety margin, dose adjustment appears to be unnecessary in subjects with mild or moderate hepatic impairment.
Copyright © 2016 Desai et al.
Figures
References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv113. - PubMed
-
- Astellas Pharma US, Inc. 2015. CRESEMBA® (isavuconazonium sulfate) prescribing information. Astellas Pharma US, Inc, Northbrook, IL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000... Accessed 10 March 2015.
-
- European Medicines Agency. 2015. Cresemba (isavuconazole). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... Accessed 11 November 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

