Leptin Induces Hypertension and Endothelial Dysfunction via Aldosterone-Dependent Mechanisms in Obese Female Mice
- PMID: 26953321
- PMCID: PMC5088432
- DOI: 10.1161/HYPERTENSIONAHA.115.06642
Leptin Induces Hypertension and Endothelial Dysfunction via Aldosterone-Dependent Mechanisms in Obese Female Mice
Abstract
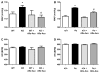
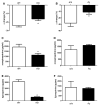
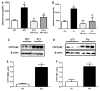
Obesity is a major risk factor for cardiovascular disease in males and females. Whether obesity triggers cardiovascular disease via similar mechanisms in both the sexes is, however, unknown. In males, the adipokine leptin highly contributes to obesity-related cardiovascular disease by increasing sympathetic activity. Females secrete 3× to 4× more leptin than males, but do not exhibit high sympathetic tone with obesity. Nevertheless, females show inappropriately high aldosterone levels that positively correlate with adiposity and blood pressure (BP). We hypothesized that leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in females. Leptin control of the cardiovascular function was analyzed in female mice sensitized to leptin via the deletion of protein tyrosine phosphatase 1b (knockout) and in agouti yellow obese hyperleptinemic mice (Ay). Hypersensitivity to leptin (wild-type, 115 ± 2; protein tyrosine phosphatase 1b knockout, 124 ± 2 mm Hg; P<0.05) and obesity elevated BP (a/a, 113 ± 1; Ay, 128 ± 7 mm Hg; P<0.05) and impaired endothelial function. Chronic leptin receptor antagonism restored BP and endothelial function in protein tyrosine phosphatase 1b knockout and Ay mice. Hypersensitivity to leptin and obesity reduced BP response to ganglionic blockade in both strains and plasma catecholamine levels in protein tyrosine phosphatase 1b knockout mice. Hypersensitivity to leptin and obesity significantly increased plasma aldosterone levels and adrenal CYP11B2 expression. Chronic leptin receptor antagonism reduced aldosterone levels. Furthermore, chronic leptin and mineralocorticoid receptor blockade reduced BP and improved endothelial function in both leptin-sensitized and obese hyperleptinemic female mice. Together, these data demonstrate that leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in female mice and suggest that obesity leads to cardiovascular disease via sex-specific mechanisms.
Keywords: hypertension; leptin; mineralocorticoid receptor; obesity; sex.
© 2016 American Heart Association, Inc.
Figures
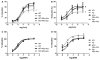

Similar articles
-
Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis.Circulation. 2015 Dec 1;132(22):2134-45. doi: 10.1161/CIRCULATIONAHA.115.018226. Epub 2015 Sep 11. Circulation. 2015. PMID: 26362633
-
Deletion of protein tyrosine phosphatase 1b in proopiomelanocortin neurons reduces neurogenic control of blood pressure and protects mice from leptin- and sympatho-mediated hypertension.Pharmacol Res. 2015 Dec;102:235-44. doi: 10.1016/j.phrs.2015.10.012. Epub 2015 Oct 30. Pharmacol Res. 2015. PMID: 26523876
-
Increasing peripheral insulin sensitivity by protein tyrosine phosphatase 1B deletion improves control of blood pressure in obesity.Hypertension. 2012 Nov;60(5):1273-9. doi: 10.1161/HYPERTENSIONAHA.112.196295. Epub 2012 Oct 8. Hypertension. 2012. PMID: 23045458 Free PMC article.
-
Leptin and Aldosterone.Vitam Horm. 2019;109:265-284. doi: 10.1016/bs.vh.2018.09.003. Epub 2018 Dec 1. Vitam Horm. 2019. PMID: 30678859 Free PMC article. Review.
-
Mineralocorticoid Receptor and Endothelial Dysfunction in Hypertension.Curr Hypertens Rep. 2019 Sep 4;21(10):78. doi: 10.1007/s11906-019-0981-4. Curr Hypertens Rep. 2019. PMID: 31485760 Free PMC article. Review.
Cited by
-
Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases.J Hum Hypertens. 2023 Aug;37(8):609-618. doi: 10.1038/s41371-022-00771-0. Epub 2022 Nov 1. J Hum Hypertens. 2023. PMID: 36319856 Free PMC article. Review.
-
Adipokines and Endothelium Dysfunction Markers in Pregnant Women with Gestational Hypertension.Int J Hypertens. 2019 Oct 13;2019:7541846. doi: 10.1155/2019/7541846. eCollection 2019. Int J Hypertens. 2019. PMID: 31737362 Free PMC article.
-
Predictive value of estimated pulse wave velocity for cardiovascular and all-cause mortality in individuals with obesity.Diabetol Metab Syndr. 2023 Mar 9;15(1):40. doi: 10.1186/s13098-023-01011-2. Diabetol Metab Syndr. 2023. PMID: 36894988 Free PMC article.
-
Endothelial cell leptin receptors, leptin and interleukin-8 in the pathogenesis of preeclampsia: An in-vitro study.Turk J Obstet Gynecol. 2017 Dec;14(4):220-227. doi: 10.4274/tjod.78545. Epub 2017 Dec 30. Turk J Obstet Gynecol. 2017. PMID: 29379664 Free PMC article.
-
The role of oxidative stress in the crosstalk between leptin and mineralocorticoid receptor in the cardiac fibrosis associated with obesity.Sci Rep. 2017 Dec 1;7(1):16802. doi: 10.1038/s41598-017-17103-9. Sci Rep. 2017. PMID: 29196758 Free PMC article.
References
-
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United states, 2011–2012. NCHS data brief. 2013:131. - PubMed
-
- Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

