Global Neuromagnetic Cortical Fields Have Non-Zero Velocity
- PMID: 26953886
- PMCID: PMC4783027
- DOI: 10.1371/journal.pone.0148413
Global Neuromagnetic Cortical Fields Have Non-Zero Velocity
Abstract
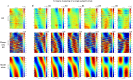
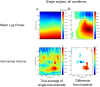
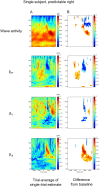
Globally coherent patterns of phase can be obscured by analysis techniques that aggregate brain activity measures across-trials, whether prior to source localization or for estimating inter-areal coherence. We analyzed, at single-trial level, whole head MEG recorded during an observer-triggered apparent motion task. Episodes of globally coherent activity occurred in the delta, theta, alpha and beta bands of the signal in the form of large-scale waves, which propagated with a variety of velocities. Their mean speed at each frequency band was proportional to temporal frequency, giving a range of 0.06 to 4.0 m/s, from delta to beta. The wave peaks moved over the entire measurement array, during both ongoing activity and task-relevant intervals; direction of motion was more predictable during the latter. A large proportion of the cortical signal, measurable at the scalp, exists as large-scale coherent motion. We argue that the distribution of observable phase velocities in MEG is dominated by spatial filtering considerations in combination with group velocity of cortical activity. Traveling waves may index processes involved in global coordination of cortical activity.
Conflict of interest statement
Figures



References
-
- Freeman WJ, Holmes MD, Burke BC, Vanhatalo S. Spatial spectra of scalp EEG and EMG from awake humans. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2003;114: 1053–1068. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

