Resting-state connectivity predicts levodopa-induced dyskinesias in Parkinson's disease
- PMID: 26954295
- PMCID: PMC5069605
- DOI: 10.1002/mds.26540
Resting-state connectivity predicts levodopa-induced dyskinesias in Parkinson's disease
Abstract
Background: Levodopa-induced dyskinesias are a common side effect of dopaminergic therapy in PD, but their neural correlates remain poorly understood.
Objectives: This study examines whether dyskinesias are associated with abnormal dopaminergic modulation of resting-state cortico-striatal connectivity.
Methods: Twelve PD patients with peak-of-dose dyskinesias and 12 patients without dyskinesias were withdrawn from dopaminergic medication. All patients received a single dose of fast-acting soluble levodopa and then underwent resting-state functional magnetic resonance imaging before any dyskinesias emerged. Levodopa-induced modulation of cortico-striatal resting-state connectivity was assessed between the putamen and the following 3 cortical regions of interest: supplementary motor area, primary sensorimotor cortex, and right inferior frontal gyrus. These functional connectivity measures were entered into a linear support vector classifier to predict whether an individual patient would develop dyskinesias after levodopa intake. Linear regression analysis was applied to test which connectivity measures would predict dyskinesia severity.
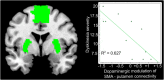
Results: Dopaminergic modulation of resting-state connectivity between the putamen and primary sensorimotor cortex in the most affected hemisphere predicted whether patients would develop dyskinesias with a specificity of 100% and a sensitivity of 91% (P < .0001). Modulation of resting-state connectivity between the supplementary motor area and putamen predicted interindividual differences in dyskinesia severity (R(2) = 0.627, P = .004). Resting-state connectivity between the right inferior frontal gyrus and putamen neither predicted dyskinesia status nor dyskinesia severity.
Conclusions: The results corroborate the notion that altered dopaminergic modulation of cortico-striatal connectivity plays a key role in the pathophysiology of dyskinesias in PD.
Keywords: Parkinson's disease; dyskinesias; fMRI; levodopa MRI.
© 2016 International Parkinson and Movement Disorder Society.
Figures


Similar articles
-
A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias.Brain. 2015 Feb;138(Pt 2):414-27. doi: 10.1093/brain/awu329. Epub 2014 Nov 19. Brain. 2015. PMID: 25414038
-
Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans.Brain. 2015 Jun;138(Pt 6):1658-66. doi: 10.1093/brain/awv096. Epub 2015 Apr 15. Brain. 2015. PMID: 25882651 Free PMC article.
-
Altered inhibitory interaction among inferior frontal and motor cortex in l-dopa-induced dyskinesias.Mov Disord. 2016 May;31(5):755-9. doi: 10.1002/mds.26520. Epub 2016 Feb 10. Mov Disord. 2016. PMID: 26861941
-
Cortical plasticity and levodopa-induced dyskinesias in Parkinson's disease: Connecting the dots in a multicomponent network.Clin Neurophysiol. 2017 Jun;128(6):992-999. doi: 10.1016/j.clinph.2017.03.043. Epub 2017 Apr 10. Clin Neurophysiol. 2017. PMID: 28454042 Review.
-
Motor fluctuations and dyskinesia in Parkinson's disease.Parkinsonism Relat Disord. 2001 Oct;8(2):101-8. doi: 10.1016/s1353-8020(01)00024-4. Parkinsonism Relat Disord. 2001. PMID: 11489675 Review.
Cited by
-
Neuroanatomical and Microglial Alterations in the Striatum of Levodopa-Treated, Dyskinetic Hemi-Parkinsonian Rats.Front Neurosci. 2020 Sep 15;14:567222. doi: 10.3389/fnins.2020.567222. eCollection 2020. Front Neurosci. 2020. PMID: 33041762 Free PMC article.
-
The study of brain functional connectivity in Parkinson's disease.Transl Neurodegener. 2016 Oct 28;5:18. doi: 10.1186/s40035-016-0066-0. eCollection 2016. Transl Neurodegener. 2016. PMID: 27800157 Free PMC article. Review.
-
Functional Connectivity Signatures of Parkinson's Disease.J Parkinsons Dis. 2019;9(4):637-652. doi: 10.3233/JPD-191592. J Parkinsons Dis. 2019. PMID: 31450512 Free PMC article. Review.
-
Dysfunction in superior frontal gyrus associated with diphasic dyskinesia in Parkinson's disease.NPJ Parkinsons Dis. 2020 Oct 30;6:30. doi: 10.1038/s41531-020-00133-y. eCollection 2020. NPJ Parkinsons Dis. 2020. PMID: 33145398 Free PMC article.
-
Cerebellar connectivity in Parkinson's disease with levodopa-induced dyskinesia.Ann Clin Transl Neurol. 2019 Nov;6(11):2251-2260. doi: 10.1002/acn3.50918. Epub 2019 Oct 23. Ann Clin Transl Neurol. 2019. PMID: 31643140 Free PMC article.
References
-
- Van Gerpen JA, Kumar N, Bower JH, Weigand S, Ahlskog JE. Levodopa‐associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976–1990. Arch Neurol 2006;63:205‐209. - PubMed
-
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700‐711. - PubMed
-
- Rowe JB, Siebner HR. The motor system and its disorders. Neuroimage 2012;61:464‐477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

