HP1-Assisted Aurora B Kinase Activity Prevents Chromosome Segregation Errors
- PMID: 26954544
- PMCID: PMC4949072
- DOI: 10.1016/j.devcel.2016.02.008
HP1-Assisted Aurora B Kinase Activity Prevents Chromosome Segregation Errors
Abstract
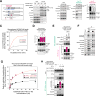
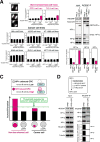
Incorrect attachment of kinetochore microtubules is the leading cause of chromosome missegregation in cancers. The highly conserved chromosomal passenger complex (CPC), containing mitotic kinase Aurora B as a catalytic subunit, ensures faithful chromosome segregation through destabilizing incorrect microtubule attachments and promoting biorientation of chromosomes on the mitotic spindle. It is unknown whether CPC dysfunction affects chromosome segregation fidelity in cancers and, if so, how. Here, we show that heterochromatin protein 1 (HP1) is an essential CPC component required for full Aurora B activity. HP1 binding to the CPC becomes particularly important when Aurora B phosphorylates kinetochore targets to eliminate erroneous microtubule attachments. Remarkably, a reduced proportion of HP1 bound to CPC is widespread in cancers, which causes an impairment in Aurora B activity. These results indicate that HP1 is an essential modulator for CPC function and identify a molecular basis for chromosome segregation errors in cancer cells.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Mitotic Mysteries: The Case of HP1.Dev Cell. 2016 Mar 7;36(5):477-8. doi: 10.1016/j.devcel.2016.02.019. Dev Cell. 2016. PMID: 26954539
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

