Cyclin-Dependent Kinase CRK9, Required for Spliced Leader trans Splicing of Pre-mRNA in Trypanosomes, Functions in a Complex with a New L-Type Cyclin and a Kinetoplastid-Specific Protein
- PMID: 26954683
- PMCID: PMC4783070
- DOI: 10.1371/journal.ppat.1005498
Cyclin-Dependent Kinase CRK9, Required for Spliced Leader trans Splicing of Pre-mRNA in Trypanosomes, Functions in a Complex with a New L-Type Cyclin and a Kinetoplastid-Specific Protein
Abstract
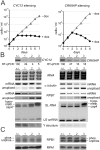
In eukaryotes, cyclin-dependent kinases (CDKs) control the cell cycle and critical steps in gene expression. The lethal parasite Trypanosoma brucei, member of the phylogenetic order Kinetoplastida, possesses eleven CDKs which, due to high sequence divergence, were generically termed CDC2-related kinases (CRKs). While several CRKs have been implied in the cell cycle, CRK9 was the first trypanosome CDK shown to control the unusual mode of gene expression found in kinetoplastids. In these organisms, protein-coding genes are arranged in tandem arrays which are transcribed polycistronically. Individual mRNAs are processed from precursor RNA by spliced leader (SL) trans splicing and polyadenylation. CRK9 ablation was lethal in cultured trypanosomes, causing a block of trans splicing before the first transesterification step. Additionally, CRK9 silencing led to dephosphorylation of RNA polymerase II and to hypomethylation of the SL cap structure. Here, we tandem affinity-purified CRK9 and, among potential CRK9 substrates and modifying enzymes, discovered an unusual tripartite complex comprising CRK9, a new L-type cyclin (CYC12) and a protein, termed CRK9-associated protein (CRK9AP), that is only conserved among kinetoplastids. Silencing of either CYC12 or CRK9AP reproduced the effects of depleting CRK9, identifying these proteins as functional partners of CRK9 in vivo. While mammalian cyclin L binds to CDK11, the CRK9 complex deviates substantially from that of CDK11, requiring CRK9AP for efficient CRK9 complex formation and autophosphorylation in vitro. Interference with this unusual CDK rescued mice from lethal trypanosome infections, validating CRK9 as a potential chemotherapeutic target.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Rapid block of pre-mRNA splicing by chemical inhibition of analog-sensitive CRK9 in Trypanosoma brucei.Mol Microbiol. 2020 Jun;113(6):1225-1239. doi: 10.1111/mmi.14489. Epub 2020 Mar 4. Mol Microbiol. 2020. PMID: 32068297 Free PMC article.
-
Trypanosome cdc2-related kinase 9 controls spliced leader RNA cap4 methylation and phosphorylation of RNA polymerase II subunit RPB1.Mol Cell Biol. 2013 May;33(10):1965-75. doi: 10.1128/MCB.00156-13. Epub 2013 Mar 11. Mol Cell Biol. 2013. PMID: 23478263 Free PMC article.
-
A divergent transcription factor TFIIB in trypanosomes is required for RNA polymerase II-dependent spliced leader RNA transcription and cell viability.Eukaryot Cell. 2006 Feb;5(2):293-300. doi: 10.1128/EC.5.2.293-300.2006. Eukaryot Cell. 2006. PMID: 16467470 Free PMC article.
-
Trans-splicing in trypanosomes: machinery and its impact on the parasite transcriptome.Future Microbiol. 2011 Apr;6(4):459-74. doi: 10.2217/fmb.11.20. Future Microbiol. 2011. PMID: 21526946 Review.
-
The response of trypanosomes and other eukaryotes to ER stress and the spliced leader RNA silencing (SLS) pathway in Trypanosoma brucei.Crit Rev Biochem Mol Biol. 2015;50(3):256-67. doi: 10.3109/10409238.2015.1042541. Epub 2015 May 19. Crit Rev Biochem Mol Biol. 2015. PMID: 25985970 Review.
Cited by
-
A distinct complex of PRP19-related and trypanosomatid-specific proteins is required for pre-mRNA splicing in trypanosomes.Nucleic Acids Res. 2021 Dec 16;49(22):12929-12942. doi: 10.1093/nar/gkab1152. Nucleic Acids Res. 2021. PMID: 34850936 Free PMC article.
-
Pedal to the Metal: Nuclear Splicing Bodies Turbo-Charge VSG mRNA Production in African Trypanosomes.Front Cell Dev Biol. 2022 Apr 20;10:876701. doi: 10.3389/fcell.2022.876701. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35517511 Free PMC article. Review.
-
Regulated protein stabilization underpins the functional interplay among basal body components in Trypanosoma brucei.J Biol Chem. 2020 Jan 17;295(3):729-742. doi: 10.1074/jbc.RA119.011352. Epub 2019 Dec 9. J Biol Chem. 2020. PMID: 31819011 Free PMC article.
-
Importance of tuberin in carcinogenesis.Oncol Lett. 2017 Sep;14(3):2598-2602. doi: 10.3892/ol.2017.6490. Epub 2017 Jun 28. Oncol Lett. 2017. PMID: 28928805 Free PMC article.
-
Atypical cyclins: the extended family portrait.Cell Mol Life Sci. 2020 Jan;77(2):231-242. doi: 10.1007/s00018-019-03262-7. Epub 2019 Aug 16. Cell Mol Life Sci. 2020. PMID: 31420702 Free PMC article. Review.
References
-
- Murphy WJ, Watkins KP, Agabian N (1986) Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell 47: 517–525. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

