Innovative farmers and regulatory gatekeepers: Genetically modified crops regulation and adoption in developing countries
- PMID: 26954893
- PMCID: PMC5033216
- DOI: 10.1080/21645698.2016.1151989
Innovative farmers and regulatory gatekeepers: Genetically modified crops regulation and adoption in developing countries
Abstract
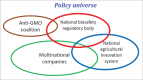
The regulation of genetically modified (GM) crops is a topical issue in agriculture and environment over the past 2 decades. The objective of this paper is to recount regulatory and adoption practices in some developing countries that have successfully adopted GM crops so that aspiring countries may draw useful lessons and best practices for their biosafatey regulatory regimes. The first 11 mega-GM crops growing countries each with an area of more than one million hectares in 2014 were examined. Only five out of the 11 countries had smooth and orderly adoption of these crops as per the regulatory requirement of each country. In the remaining 6 countries (all developing countries), GM crops were either introduced across borders without official authorization, released prior to regulatory approval or unapproved seeds were sold along with the approved ones in violation to the existing regulations. Rapid expansion of transgenic crops over the past 2 decades in the developing world was a result of an intense desire by farmers to adopt these crops irrespective of regulatory roadblocks. Lack of workable biosafety regulatory system and political will to support GM crops encouraged unauthorized access to GM crop varieties. In certain cases, unregulated access in turn appeared to result in the adoption of substandard or spurious technology which undermined performance and productivity. An optimal interaction among the national agricultural innovation systems, biosafety regulatory bodies, biotech companies and high level policy makers is vital in making a workable regulated progress in the adoption of GM crops. Factoring forgone opportunities to farmers to benefit from GM crops arising from overregulation into biosafety risk analysis and decision making is suggested. Building functional biosafety regulatory systems that balances the needs of farmers to access and utilize the GM technology with the regulatory imperatives to ensure adequate safety to the environment and human health is recommended.
Keywords: GM crops adoption history; biosafety regulation; functional biosafety systems; unauthorized release.
Figures
Similar articles
-
Genetically modified crops and small-scale farmers: main opportunities and challenges.Crit Rev Biotechnol. 2016;36(3):434-46. doi: 10.3109/07388551.2014.990413. Epub 2015 Jan 8. Crit Rev Biotechnol. 2016. PMID: 25566797 Review.
-
The release of genetically modified crops into the environment. Part I. Overview of current status and regulations.Plant J. 2003 Jan;33(1):1-18. doi: 10.1046/j.0960-7412.2003.01602.x. Plant J. 2003. PMID: 12943538 Review.
-
Status of market, regulation and research of genetically modified crops in Chile.N Biotechnol. 2016 Dec 25;33(6):815-823. doi: 10.1016/j.nbt.2016.07.017. Epub 2016 Jul 26. N Biotechnol. 2016. PMID: 27474111 Review.
-
Economic impacts of policies affecting crop biotechnology and trade.N Biotechnol. 2010 Nov 30;27(5):558-64. doi: 10.1016/j.nbt.2010.05.012. Epub 2010 May 15. N Biotechnol. 2010. PMID: 20478422 Review.
-
Regulatory challenges for GM crops in developing economies: the African experience.Transgenic Res. 2014 Dec;23(6):1049-55. doi: 10.1007/s11248-014-9805-0. Epub 2014 May 13. Transgenic Res. 2014. PMID: 24821674
Cited by
-
Biosafety regulatory frameworks in Kenya, Nigeria, Uganda and Sweden and their potential impact on international R&D collaborations.GM Crops Food. 2023 Dec 31;14(1):1-17. doi: 10.1080/21645698.2023.2194221. GM Crops Food. 2023. PMID: 36987578 Free PMC article.
-
Crop Biotechnology and Product Stewardship.GM Crops Food. 2021 Jan 2;12(1):106-114. doi: 10.1080/21645698.2020.1822133. GM Crops Food. 2021. PMID: 33079624 Free PMC article.
-
The Search for Resistance to Cassava Mosaic Geminiviruses: How Much We Have Accomplished, and What Lies Ahead.Front Plant Sci. 2017 Mar 24;8:408. doi: 10.3389/fpls.2017.00408. eCollection 2017. Front Plant Sci. 2017. PMID: 28392798 Free PMC article. Review.
-
Regulation of genome edited technologies in India.Transgenic Res. 2019 Aug;28(Suppl 2):175-181. doi: 10.1007/s11248-019-00148-z. Transgenic Res. 2019. PMID: 31321702
-
Advancements in genetic studies of mushrooms: a comprehensive review.World J Microbiol Biotechnol. 2024 Jul 22;40(9):275. doi: 10.1007/s11274-024-04079-8. World J Microbiol Biotechnol. 2024. PMID: 39034336 Review.
References
-
- Barton K.A., and Brill W.J.. Prospects in plant genetic engineering. Science 1983; 219:671-676; PMID:6297007; http://dx.doi.org/10.1126/science.6297007 - DOI - PubMed
-
- Berg P., Baltimore D., Boyer H.W., Cohen S.N., Davis R.W., Hogness D.S., Nathans D., Roblin R., Watson J.D., Weissman S., and Zinder N.D.. Potential biohazards of recombinant DNA molecules. Science 1974; 185:303; http://dx.doi.org/10.1126/science.185.4148.303 - DOI - PubMed
-
- Berg P., Baltimore D., Brenner S., Roblin R.O., and Singer M.F.. Asilomar Conference on Recombinant DNA Molecules. Science 1975; 18:991-994; http://dx.doi.org/10.1126/science.1056638 - DOI - PubMed
-
- CBD (Secretariat of the Convention on Biological Diversity) Cartagena Protocol on Biosafety to the Convention on Biological Diversity: text and annexes Montreal: Secretariat of the Convention on Biological Diversity. 2000. Available from: https://www.cbd.int/doc/legal/cartagena-protocol-en.pdf
-
- CBD (Secretariat of the Convention on Biological Diversity) The Nagoya - Kuala Lumpur Supplementary Protocol on Liability and Redress to the Cartagena Protocol on Biosafety. Montreal, Quebec, Canada: 2011. Available from: https://bch.cbd.int/protocol/supplementary/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous