A Structural Basis for How Motile Cilia Beat
- PMID: 26955066
- PMCID: PMC4776691
- DOI: 10.1093/biosci/biu180
A Structural Basis for How Motile Cilia Beat
Abstract
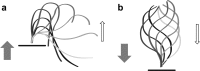
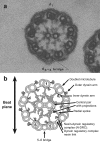
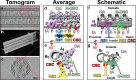
The motile cilium is a mechanical wonder, a cellular nanomachine that produces a high-speed beat based on a cycle of bends that move along an axoneme made of 9+2 microtubules. The molecular motors, dyneins, power the ciliary beat. The dyneins are compacted into inner and outer dynein arms, whose activity is highly regulated to produce microtubule sliding and axonemal bending. The switch point hypothesis was developed long ago to account for how sliding in the presence of axonemal radial spoke-central pair interactions causes the ciliary beat. Since then, a new genetic, biochemical, and structural complexity has been discovered, in part, with Chlamydomonas mutants, with high-speed, high-resolution analysis of movement and with cryoelectron tomography. We stand poised on the brink of new discoveries relating to the molecular control of motility that extend and refine our understanding of the basic events underlying the switching of arm activity and of bend formation and propagation.
Keywords: axoneme; cilia; dynein; eukaryotic flagella; microtubules; motility.
Figures

References
-
- Blake JR, Sleigh MA. Mechanics of ciliary locomotion. Biological Reviews of the Cambridge Philosophical Society. 1974;49:85–125. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

