Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study
- PMID: 26955082
- PMCID: PMC4776721
- DOI: 10.1093/biosci/biv084
Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study
Abstract
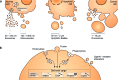
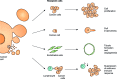
The release of extracellular vesicles (EVs), including exosomes and microvesicles, is a phenomenon shared by many cell types as a means of communicating with other cells and also potentially removing cell contents. The cargo of EVs includes the proteins, lipids, nucleic acids, and membrane receptors of the cells from which they originate. EVs released into the extracellular space can enter body fluids and potentially reach distant tissues. Once taken up by neighboring and/or distal cells, EVs can transfer functional cargo that may alter the status of recipient cells, thereby contributing to both physiological and pathological processes. In this article, we will focus on EV composition, mechanisms of uptake, and their biological effects on recipient cells. We will also discuss established and recently developed methods used to study EVs, including isolation, quantification, labeling and imaging protocols, as well as RNA analysis.
Keywords: exosomes; extracellular vesicles; intercellular communication; methods; microvesicles.
Figures

References
-
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature Cell Biology. 2008;10:619–624. - PubMed
-
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell–derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells; Proceedings of the National Academy of Sciences; 2011. pp. 4852–4857. - PMC - PubMed
-
- Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Affabris E, Baur A, Federico M. Exosomes from human immunodeficiency virus type 1 (HIV-1)–infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. Journal of Virology. 2014;88:11529–11539. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

