Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil
- PMID: 26955192
- PMCID: PMC4779794
- DOI: 10.1016/j.simyco.2014.11.002
Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil
Abstract
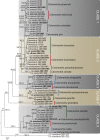
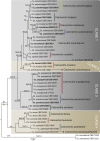
Species in the genus Calonectria (Hypocreales) represent an important group of plant pathogenic fungi that cause serious losses to plant crops in tropical and subtropical climates. Calonectria leaf blight is currently one of the main impediments to Eucalyptus cultivation in Brazil, and various species of Calonectria have been associated with this disease. Since most previous identifications were solely based on morphological characters, much of the published literature needs to be re-evaluated. The aim of this study was thus to identify and determine the phylogenetic relationships among species that occur in the Eucalyptus growing regions of Brazil by using partial sequences of the β-tubulin, calmodulin, translation elongation factor 1-α and histone H3 gene regions. Based on extensive collections from soil and infected eucalypt leaf samples from plantations, phylogenetic inference revealed the Ca. pteridis complex to be the most common species complex present in Eucalyptus plantations in Brazil. By elucidating taxa in the Ca. pteridis, Ca. cylindrospora and Ca. candelabra species complexes, 20 novel Calonectria species were identified, and a new name in Calonectria provided for Cylindrocladium macrosporum as Ca. pseudopteridis.
Keywords: Ca. duoramosa R.F. Alfenas, L. Lombard & Crous; Ca. eucalypticola R.F. Alfenas, L. Lombard & Crous; Ca. glaebicola R.F. Alfenas, L. Lombard & Crous; Ca. maranhensis R.F. Alfenas, L. Lombard & Crous; Ca. metrosideri R.F. Alfenas, O.L. Pereira, Crous & A.C. Alfenas; Ca. multinaviculata R.F. Alfenas, L. Lombard & Crous; Ca. nemuricola R.F. Alfenas, L. Lombard & Crous; Ca. paraensis R.F. Alfenas, L. Lombard & Crous; Ca. piauiensis R.F. Alfenas, L. Lombard & Crous; Ca. propaginicola R.F. Alfenas, L. Lombard & Crous; Ca. pseudobrassicae R.F. Alfenas, L. Lombard & Crous; Ca. pseudocerciana R.F. Alfenas, L. Lombard & Crous; Ca. pseudohodgesii R.F. Alfenas, L. Lombard & Crous; Ca. pseudometrosideri R.F. Alfenas, L. Lombard & Crous; Ca. pseudopteridis (Sherb.) R.F. Alfenas, L. Lombard & Crous; Ca. pseudospathulata R.F. Alfenas, L. Lombard & Crous; Ca. pseudovata R.F. Alfenas, L. Lombard & Crous; Ca. quinqueramosa R.F. Alfenas, L. Lombard & Crous; Ca. robigophila R.F. Alfenas, L. Lombard & Crous; Ca. silvicola R.F. Alfenas, L. Lombard & Crous; Ca. telluricola R.F. Alfenas, L. Lombard & Crous; Calonectria brassiana R.F. Alfenas, L. Lombard & Crous; Calonectria leaf blight; Cylindrocladium; Damping-off; Diversity; Taxonomy.
Figures






















References
-
- Ahmad N., Ahmad S. Needle blight disease of pine caused by Cylindrocladium macrosporum. Malaysian Forester. 1982;45:84–86.
-
- Alfenas A.C. Fungos do gênero Cylindrocladium como patógenos florestais, no Brasil. Fitopatologia Brasileira. 1986;11:275–277.
-
- Alfenas A.C., Zauza E.A.V., Mafia R.G. 2nd. ed. Editora UFV; Viçosa, MG, Brazil: 2009. Clonagem e doenças do eucalipto.
-
- Alfenas A.C., Ferreira F.A. A mancha de folha do eucalipto no Brasil causada por três espécies de Cylindrocladium – Uma revisão da descrição da doença. Revista Árvore. 1979;3:47–56.
-
- Alfenas A.C., Matsuoka A.K., Ferreira F.A. Identificação, características culturais e patogenicidade de três espécies de Cylindrocladium, isoladas de manchas de folha de Eucalyptus spp. Fitopatologia Brasileira. 1979;4:445–459.
LinkOut - more resources
Full Text Sources
Other Literature Sources
