Pathogenesis and Diagnostic Approaches of Avian Infectious Bronchitis
- PMID: 26955391
- PMCID: PMC4756178
- DOI: 10.1155/2016/4621659
Pathogenesis and Diagnostic Approaches of Avian Infectious Bronchitis
Abstract
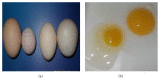
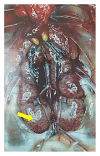
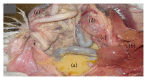
Infectious bronchitis (IB) is one of the major economically important poultry diseases distributed worldwide. It is caused by infectious bronchitis virus (IBV) and affects both galliform and nongalliform birds. Its economic impact includes decreased egg production and poor egg quality in layers, stunted growth, poor carcass weight, and mortality in broiler chickens. Although primarily affecting the respiratory tract, IBV demonstrates a wide range of tissues tropism, including the renal and reproductive systems. Thus, disease outcome may be influenced by the organ or tissue involved as well as pathotypes or strain of the infecting virus. Knowledge on the epidemiology of the prevalent IBV strains in a particular region is therefore important to guide control and preventions. Meanwhile previous diagnostic methods such as serology and virus isolations are less sensitive and time consuming, respectively; current methods, such as reverse transcription polymerase chain reaction (RT-PCR), Restriction Fragment Length Polymorphism (RFLP), and sequencing, offer highly sensitive, rapid, and accurate diagnostic results, thus enabling the genotyping of new viral strains within the shortest possible time. This review discusses aspects on pathogenesis and diagnostic methods for IBV infection.
Figures






References
-
- Arshad S. S. Infectious bronchitis. In: Zamri-Saad M., editor. Diseases of Poultry in South East Asia. Serdang, Malaysia: Universiti Putra Malaysia Press; 2006. pp. 199–206.
-
- Schalk A., Hawn M. An apparently new respiratory disease of baby chicks. Journal of the American Veterinary Medical Association. 1931;78:413–422.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

