Nanomedicines for Endothelial Disorders
- PMID: 26955397
- PMCID: PMC4778260
- DOI: 10.1016/j.nantod.2015.11.009
Nanomedicines for Endothelial Disorders
Abstract
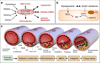
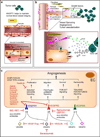
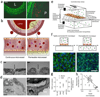
The endothelium lines the internal surfaces of blood and lymphatic vessels and has a critical role in maintaining homeostasis. Endothelial dysfunction is involved in the pathology of many diseases and conditions, including disorders such as diabetes, cardiovascular diseases, and cancer. Given this common etiology in a range of diseases, medicines targeting an impaired endothelium can strengthen the arsenal of therapeutics. Nanomedicine - the application of nanotechnology to healthcare - presents novel opportunities and potential for the treatment of diseases associated with an impaired endothelium. This review discusses therapies currently available for the treatment of these disorders and highlights the application of nanomedicine for the therapy of these major disease complications.
Keywords: atherosclerosis; cancer; diabetes; endothelial disorder; endothelium; nanomedicine; permeability.
Conflict of interest statement
R.L. and O.C.F. have financial interests in BIND Therapeutics, Selecta Biosciences, and Blend Therapeutics, which are developing nanoparticle technologies for medical applications. These companies did not support the aforementioned research and currently have no rights to any technology or intellectual property developed as part of this research. All other authors declare no conflicts.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
