The use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer
- PMID: 26955657
- PMCID: PMC4778250
- DOI: 10.1021/acscentsci.5b00121
The use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer
Abstract
We generated 18F-labeled antibody fragments for PET imaging using a sortase-mediated reaction to install a transcyclooctene (TCO)-functionalized short peptide onto proteins of interest, followed by reaction with a tetrazine-labeled-18F-2-deoxyfluoroglucose (FDG). The method is rapid, robust, and site-specific (radiochemical yields >25%, not decay corrected). The availability of 18F-2-deoxyfluoroglucose avoids the need for more complicated chemistries used to generate carbon-fluorine bonds. We demonstrate the utility of the method by detecting heterotopic pancreatic tumors in mice by PET, using anti-Class II MHC single domain antibodies. We correlate macroscopic PET images with microscopic two-photon visualization of the tumor. Our approach provides easy access to 18F-labeled antibodies and their fragments at a level of molecular specificity that complements conventional18F-FDG imaging.
Figures


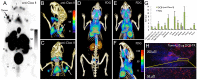
References
-
- Quigley H.; Colloby S. J.; O’Brien J. T. PET imaging of brain amyloid in dementia: a review. Int. J. Geriatr. Psychiatry 2011, 2610991–999. - PubMed
-
- Dijkers E. C.; et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 875586–592. - PubMed
-
- Pichler B. J.; Kolb A.; Nägele T.; Schlemmer H.-P. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J. Nucl. Med. 2010, 513333–336. - PubMed
-
- Schlemmer H. P.; Pichler B. J.; Schmand M.; Burbar Z.; Michel C.; Ladebeck R.; Jattke K.; Townsend D.; Nahmias C.; Jacob P. K.; Heiss W. D.; Claussen C. D. Simultaneous MR/PET Imaging of the Human Brain: Feasibility Study. Radiology 2008, 24831028–1035. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
