Impaired Angiogenic Potential of Human Placental Mesenchymal Stromal Cells in Intrauterine Growth Restriction
- PMID: 26956210
- PMCID: PMC4798734
- DOI: 10.5966/sctm.2015-0155
Impaired Angiogenic Potential of Human Placental Mesenchymal Stromal Cells in Intrauterine Growth Restriction
Abstract
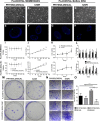
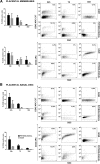
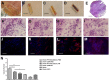
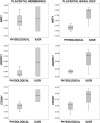
Human placental mesenchymal stromal cells (pMSCs) have never been investigated in intrauterine growth restriction (IUGR). We characterized cells isolated from placental membranes and the basal disc of six IUGR and five physiological placentas. Cell viability and proliferation were assessed every 7 days during a 6-week culture. Expression of hematopoietic, stem, endothelial, and mesenchymal markers was evaluated by flow cytometry. We characterized the multipotency of pMSCs and the expression of genes involved in mitochondrial content and function. Cell viability was high in all samples, and proliferation rate was lower in IUGR compared with control cells. All samples presented a starting heterogeneous population, shifting during culture toward homogeneity for mesenchymal markers and occurring earlier in IUGR than in controls. In vitro multipotency of IUGR-derived pMSCs was restricted because their capacity for adipocyte differentiation was increased, whereas their ability to differentiate toward endothelial cell lineage was decreased. Mitochondrial content and function were higher in IUGR pMSCs than controls, possibly indicating a shift from anaerobic to aerobic metabolism, with the loss of the metabolic characteristics that are typical of undifferentiated multipotent cells.
Significance: This study demonstrates that the loss of endothelial differentiation potential and the increase of adipogenic ability are likely to play a significant role in the vicious cycle of abnormal placental development in intrauterine growth restriction (IUGR). This is the first observation of a potential role for placental mesenchymal stromal cells in intrauterine growth restriction, thus leading to new perspectives for the treatment of IUGR.
Keywords: Intrauterine growth restriction; Mesenchymal stromal cells; Placenta; Pregnancy; Stem cells.
©AlphaMed Press.
Figures



Similar articles
-
Mesenchymal Stem/Stromal Cells from the Placentae of Growth Restricted Pregnancies Are Poor Stimulators of Angiogenesis.Stem Cell Rev Rep. 2020 Jun;16(3):557-568. doi: 10.1007/s12015-020-09959-8. Stem Cell Rev Rep. 2020. PMID: 32080795
-
Fibrocyte-like cells from intrauterine growth restriction placentas have a reduced ability to stimulate angiogenesis.Am J Pathol. 2013 Sep;183(3):1025-33. doi: 10.1016/j.ajpath.2013.06.007. Epub 2013 Jul 5. Am J Pathol. 2013. PMID: 23835310
-
Endothelial colony-forming cells derived from pregnancies complicated by intrauterine growth restriction are fewer and have reduced vasculogenic capacity.J Clin Endocrinol Metab. 2013 Dec;98(12):4953-60. doi: 10.1210/jc.2013-2580. Epub 2013 Oct 8. J Clin Endocrinol Metab. 2013. PMID: 24106289 Free PMC article.
-
Telomere homeostasis in IUGR placentas - A review.Placenta. 2016 Mar;39:21-3. doi: 10.1016/j.placenta.2015.11.006. Epub 2016 Jan 2. Placenta. 2016. PMID: 26992670 Review.
-
Can we fix it? Evaluating the potential of placental stem cells for the treatment of pregnancy disorders.Placenta. 2014 Feb;35(2):77-84. doi: 10.1016/j.placenta.2013.12.010. Epub 2013 Dec 30. Placenta. 2014. PMID: 24406265 Review.
Cited by
-
Implications of maternal-fetal health on perinatal stem cell banking.Gene Ther. 2024 Mar;31(3-4):65-73. doi: 10.1038/s41434-023-00426-w. Epub 2023 Oct 26. Gene Ther. 2024. PMID: 37880336 Free PMC article. Review.
-
Impact of Obesity and Hyperglycemia on Placental Mitochondria.Oxid Med Cell Longev. 2018 Aug 14;2018:2378189. doi: 10.1155/2018/2378189. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30186542 Free PMC article.
-
Altered expression of G1/S phase cell cycle regulators in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise.Cell Cycle. 2017 Jan 17;16(2):200-212. doi: 10.1080/15384101.2016.1261766. Epub 2016 Dec 12. Cell Cycle. 2017. PMID: 27937072 Free PMC article.
-
Placental ESRRG-CYP19A1 Expressions and Circulating 17-Beta Estradiol in IUGR Pregnancies.Front Pediatr. 2019 Apr 24;7:154. doi: 10.3389/fped.2019.00154. eCollection 2019. Front Pediatr. 2019. PMID: 31069202 Free PMC article.
-
Effect of Placenta-Derived Mesenchymal Stromal Cells Conditioned Media on an LPS-Induced Mouse Model of Preeclampsia.Int J Mol Sci. 2022 Jan 31;23(3):1674. doi: 10.3390/ijms23031674. Int J Mol Sci. 2022. PMID: 35163594 Free PMC article.
References
-
- Nuzzo AM, Giuffrida D, Zenerino C, et al. JunB/cyclin-D1 imbalance in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise. Placenta. 2014;35:483–490. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
-
- Brooke G, Tong H, Levesque JP, et al. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17:929–940. - PubMed
-
- Yen BL, Huang HI, Chien CC, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. - PubMed
-
- Abumaree MH, Al Jumah MA, Kalionis B, et al. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013;9:16–31. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

