Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems
- PMID: 26956246
- PMCID: PMC4813427
- DOI: 10.1124/pr.114.009209
Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems
Abstract
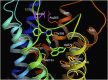
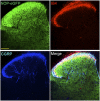
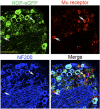
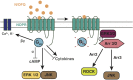
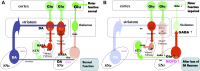
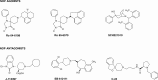
The NOP receptor (nociceptin/orphanin FQ opioid peptide receptor) is the most recently discovered member of the opioid receptor family and, together with its endogenous ligand, N/OFQ, make up the fourth members of the opioid receptor and opioid peptide family. Because of its more recent discovery, an understanding of the cellular and behavioral actions induced by NOP receptor activation are less well developed than for the other members of the opioid receptor family. All of these factors are important because NOP receptor activation has a clear modulatory role on mu opioid receptor-mediated actions and thereby affects opioid analgesia, tolerance development, and reward. In addition to opioid modulatory actions, NOP receptor activation has important effects on motor function and other physiologic processes. This review discusses how NOP pharmacology intersects, contrasts, and interacts with the mu opioid receptor in terms of tertiary structure and mechanism of receptor activation; location of receptors in the central nervous system; mechanisms of desensitization and downregulation; cellular actions; intracellular signal transduction pathways; and behavioral actions with respect to analgesia, tolerance, dependence, and reward. This is followed by a discussion of the agonists and antagonists that have most contributed to our current knowledge. Because NOP receptors are highly expressed in brain and spinal cord and NOP receptor activation sometimes synergizes with mu receptor-mediated actions and sometimes opposes them, an understanding of NOP receptor pharmacology in the context of these interactions with the opioid receptors will be crucial to the development of novel therapeutics that engage the NOP receptor.
U.S. Government work not protected by U.S. copyright.
Figures

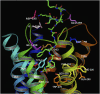
References
-
- Aguila B, Simaan M, Laporte SA. (2011) Study of G protein-coupled receptor/β-arrestin interactions within endosomes using FRAP. Methods Mol Biol 756:371–380. - PubMed
-
- Akuzawa N, Takeda S, Ishiguro M. (2007) Structural modelling and mutation analysis of a nociceptin receptor and its ligand complexes. J Biochem 141:907–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

