Tissue-Specific Cultured Human Pericytes: Perivascular Cells from Smooth Muscle Tissue Have Restricted Mesodermal Differentiation Ability
- PMID: 26956507
- PMCID: PMC4854214
- DOI: 10.1089/scd.2015.0336
Tissue-Specific Cultured Human Pericytes: Perivascular Cells from Smooth Muscle Tissue Have Restricted Mesodermal Differentiation Ability
Abstract
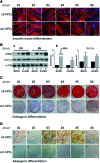
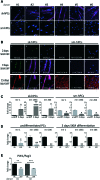
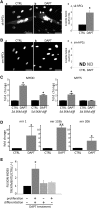
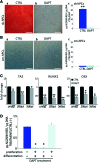
Microvascular pericytes (PCs) are considered the adult counterpart of the embryonic mesoangioblasts, which represent a multipotent cell population that resides in the dorsal aorta of the developing embryo. Although PCs have been isolated from several adult organs and tissues, it is still controversial whether PCs from different tissues exhibit distinct differentiation potentials. To address this point, we investigated the differentiation potentials of isogenic human cultured PCs isolated from skeletal (sk-hPCs) and smooth muscle tissues (sm-hPCs). We found that both sk-hPCs and sm-hPCs expressed known pericytic markers and did not express endothelial, hematopoietic, and myogenic markers. Both sk-hPCs and sm-hPCs were able to differentiate into smooth muscle cells. In contrast, sk-hPCs, but not sm-hPCs, differentiated in skeletal muscle cells and osteocytes. Given the reported ability of the Notch pathway to regulate skeletal muscle and osteogenic differentiation, sk-hPCs and sm-hPCs were treated with N-[N-(3,5- difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a known inhibitor of Notch signaling. DAPT treatment, as assessed by histological and molecular analysis, enhanced myogenic differentiation and abolished osteogenic potential of sk-hPCs. In contrast, DAPT treatment did not affect either myogenic or osteogenic differentiation of sm-hPCs. In summary, these results indicate that, despite being isolated from the same anatomical niche, cultured PCs from skeletal muscle and smooth muscle tissues display distinct differentiation abilities.
Figures

Similar articles
-
A novel population of local pericyte precursor cells in tumor stroma that require Notch signaling for differentiation.Microvasc Res. 2015 Sep;101:38-47. doi: 10.1016/j.mvr.2015.05.004. Epub 2015 Jun 17. Microvasc Res. 2015. PMID: 26092680
-
Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity.Stem Cells. 2015 Feb;33(2):557-73. doi: 10.1002/stem.1868. Stem Cells. 2015. PMID: 25336400 Free PMC article.
-
Human pericytes isolated from adipose tissue have better differentiation abilities than their mesenchymal stem cell counterparts.Cell Tissue Res. 2015 Sep;361(3):769-78. doi: 10.1007/s00441-015-2166-z. Epub 2015 Mar 29. Cell Tissue Res. 2015. PMID: 25820673
-
Pericytes in Skeletal Muscle.Adv Exp Med Biol. 2019;1122:59-72. doi: 10.1007/978-3-030-11093-2_4. Adv Exp Med Biol. 2019. PMID: 30937863 Review.
-
Pericytes: Properties, Functions and Applications in Tissue Engineering.Stem Cell Rev Rep. 2015 Aug;11(4):549-59. doi: 10.1007/s12015-015-9590-z. Stem Cell Rev Rep. 2015. PMID: 25865146 Review.
Cited by
-
Abnormalities in Skeletal Muscle Myogenesis, Growth, and Regeneration in Myotonic Dystrophy.Front Neurol. 2018 May 28;9:368. doi: 10.3389/fneur.2018.00368. eCollection 2018. Front Neurol. 2018. PMID: 29892259 Free PMC article. Review.
-
Ameliorated cellular hallmarks of myotonic dystrophy in hybrid myotubes from patient and unaffected donor cells.Stem Cell Res Ther. 2024 Sep 15;15(1):302. doi: 10.1186/s13287-024-03913-y. Stem Cell Res Ther. 2024. PMID: 39278936 Free PMC article.
-
Systemic cell therapy for muscular dystrophies : The ultimate transplantable muscle progenitor cell and current challenges for clinical efficacy.Stem Cell Rev Rep. 2021 Jun;17(3):878-899. doi: 10.1007/s12015-020-10100-y. Epub 2020 Dec 21. Stem Cell Rev Rep. 2021. PMID: 33349909 Free PMC article. Review.
-
Ether-Oxygen Containing Electrospun Microfibrous and Sub-Microfibrous Scaffolds Based on Poly(butylene 1,4-cyclohexanedicarboxylate) for Skeletal Muscle Tissue Engineering.Int J Mol Sci. 2018 Oct 17;19(10):3212. doi: 10.3390/ijms19103212. Int J Mol Sci. 2018. PMID: 30336625 Free PMC article.
-
Non-fibro-adipogenic pericytes from human embryonic stem cells attenuate degeneration of the chronically injured mouse muscle.JCI Insight. 2019 Dec 19;4(24):e125334. doi: 10.1172/jci.insight.125334. JCI Insight. 2019. PMID: 31852842 Free PMC article.
References
-
- Da Silva Meirelles L, Chagastelles PC. and Nardi NB. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119:2204–2213 - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 - PubMed
-
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP. and Hedrick MH. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228 - PubMed
-
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, et al. (2007). Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow). Blood 110:3438–3446 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

