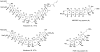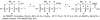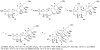Synthesis of the ABCDEF and FGHI ring system of yessotoxin and adriatoxin
- PMID: 26956788
- PMCID: PMC4898783
- DOI: 10.1038/ja.2016.18
Synthesis of the ABCDEF and FGHI ring system of yessotoxin and adriatoxin
Abstract
Yessotoxin and adriatoxin are members of the polycyclic ether family of marine natural products. Outlined in this article is our synthetic approach to two subunits of these targets. Central to our strategy is a coupling sequence that employs an olefinic-ester cyclization reaction. As outlined, this sequence was used in two coupling sequences. First, it was used to merge the A, B- and E, F-bicyclic precursors and in the process generate the C- and D-rings. Second, it was used to couple the F- and I-rings while building the eight-membered G-ring and subsequently the H-ring pyran.
Figures
Similar articles
-
Efficient Synthesis of Fused Polycyclic Ether Systems via Sulfonium Ylides: A Synthetic Approach to Yessotoxin and Adriatoxin.Mar Drugs. 2025 Jan 21;23(2):51. doi: 10.3390/md23020051. Mar Drugs. 2025. PMID: 39997175 Free PMC article.
-
Sulfonyl-stabilized oxiranyllithium-based approach to polycyclic ethers. Convergent synthesis of the ABCDEF-ring system of yessotoxin and adriatoxin.J Org Chem. 2003 Nov 14;68(23):9050-60. doi: 10.1021/jo035145d. J Org Chem. 2003. PMID: 14604380
-
Synthetic studies on maitotoxin. 3. Stereoselective synthesis of the BCDE-ring system.Org Lett. 2008 May 1;10(9):1683-5. doi: 10.1021/ol8002699. Epub 2008 Apr 8. Org Lett. 2008. PMID: 18393509
-
[Convergent Synthesis of Fused Ring Systems in Large Polycyclic Ethers].Yakugaku Zasshi. 2017;137(9):1095-1101. doi: 10.1248/yakushi.17-00116. Yakugaku Zasshi. 2017. PMID: 28867696 Review. Japanese.
-
Recent advances in the synthesis of marine polycyclic ether natural products.Curr Opin Drug Discov Devel. 2007 Nov;10(6):784-806. Curr Opin Drug Discov Devel. 2007. PMID: 17987529 Review.
Cited by
-
Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches.Mar Drugs. 2021 Jan 31;19(2):78. doi: 10.3390/md19020078. Mar Drugs. 2021. PMID: 33572583 Free PMC article. Review.
-
Biotechnological and Pharmacological Applications of Biotoxins and Other Bioactive Molecules from Dinoflagellates.Mar Drugs. 2017 Dec 20;15(12):393. doi: 10.3390/md15120393. Mar Drugs. 2017. PMID: 29261163 Free PMC article. Review.
-
Efficient Synthesis of Fused Polycyclic Ether Systems via Sulfonium Ylides: A Synthetic Approach to Yessotoxin and Adriatoxin.Mar Drugs. 2025 Jan 21;23(2):51. doi: 10.3390/md23020051. Mar Drugs. 2025. PMID: 39997175 Free PMC article.
-
Synthetic efforts towards the stereoselective synthesis of NF00659B1.Bioorg Med Chem Lett. 2018 Sep 1;28(16):2746-2750. doi: 10.1016/j.bmcl.2018.02.038. Epub 2018 Feb 19. Bioorg Med Chem Lett. 2018. PMID: 29503022 Free PMC article.
References
-
- Murata M, Kumagai M, Masanori K, Lee JS, Yasumoto T. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 1987;28:5869–5872.
-
- Satake M, MacKenzie L, Yasumoto T. Identification of Protoceratium reticulatum as the biogenetic origin of yessotoxin. Nat Toxins. 1997;5:164–167. - PubMed
-
- Paz B, Riobó P, Fernández LM, Fraga S, Franco JM. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon. 2004;44:251–258. - PubMed
-
- Rhodes L, McNabb P, de Salas M, Briggs L, Beuzenberg V, Gladstone M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae. 2006;5:148–155.
-
- Tubaro A, Dell’Ovo V, Sosa S, Florio C. Yessotoxins: A toxicological overview. Toxicon. 2010;56:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials