A multicenter randomized controlled open-label trial to assess the efficacy of compound kushen injection in combination with single-agent chemotherapy in treatment of elderly patients with advanced non-small cell lung cancer: study protocol for a randomized controlled trial
- PMID: 26956875
- PMCID: PMC4782312
- DOI: 10.1186/s13063-016-1231-6
A multicenter randomized controlled open-label trial to assess the efficacy of compound kushen injection in combination with single-agent chemotherapy in treatment of elderly patients with advanced non-small cell lung cancer: study protocol for a randomized controlled trial
Abstract
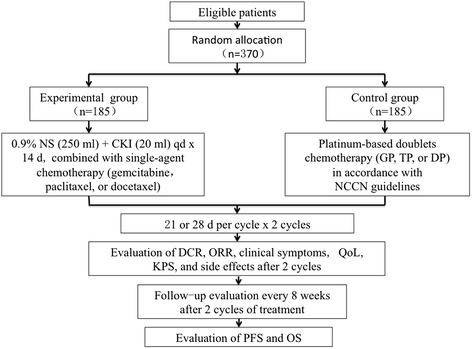
Background: With the aging of the global population, an increasing number of elderly are diagnosed with advanced non-small cell lung cancer. Although systematic chemotherapy has been one of the primary treatments for advanced non-small cell lung cancer worldwide, the elderly cannot always tolerate standard platinum-based doublet chemotherapy, thus resulting in treatment failure. To reduce toxicity, single-agent chemotherapy is often used to treat the elderly with non-small cell lung cancer; however, this may increase the risk of treatment failure due to an inadequate dose. It has been shown that compound kushen injection in combination with chemotherapy can enhance the efficacy and reduce the toxicity. The aim of this trial is to assess the clinical effectiveness and safety of compound kushen injection in combination with single-agent chemotherapy versus platinum-based doublet chemotherapy in the treatment of elderly patients with advanced non-small cell lung cancer.
Methods: This multicenter study will be an open-label, randomized controlled trial. Three hundred seventy elderly patients with advanced non-small cell lung cancer will be randomly divided into experimental (n = 185) and control groups (n = 185) to receive compound kushen injection in combination with single-agent chemotherapy or standard platinum-based doublet chemotherapy for two cycles. After two cycles, the disease control rate, objective response rate, clinical symptoms, quality of life, Karnofsky Performance Status, and side effects will be assessed. Follow-up evaluations will be performed every 8 weeks to evaluate the progression-free and overall survival.
Discussion: Before the trial was designed, compound kushen injection was shown to be effective for lung cancer through basic experiments and clinical trials. This study will determine whether or not the efficacy of compound kushen injection in combination with single-agent chemotherapy is comparable to that of platinum-based doublet chemotherapy, and whether or not the toxicity of compound kushen injection in combination with single-agent chemotherapy is lower than that of platinum-based doublet chemotherapy.
Trial registration: ChiCTR-IPR-14005484 (16 November 2014).
Similar articles
-
Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study.Lancet Oncol. 2008 Dec;9(12):1135-42. doi: 10.1016/S1470-2045(08)70261-4. Epub 2008 Nov 13. Lancet Oncol. 2008. PMID: 19013107 Clinical Trial.
-
Assessing the effectiveness and safety of liposomal paclitaxel in combination with cisplatin as first-line chemotherapy for patients with advanced NSCLC with regional lymph-node metastasis: study protocol for a randomized controlled trial (PLC-GC trial).Trials. 2013 Feb 15;14:45. doi: 10.1186/1745-6215-14-45. Trials. 2013. PMID: 23413951 Free PMC article. Clinical Trial.
-
Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial.Lancet Oncol. 2012 Mar;13(3):239-46. doi: 10.1016/S1470-2045(11)70393-X. Epub 2012 Jan 26. Lancet Oncol. 2012. PMID: 22285168 Clinical Trial.
-
Does chemotherapy have a role as palliative therapy for unfit or elderly patients with non-small-cell lung cancer?Lung Cancer. 2002 Nov;38 Suppl 2:S45-50. doi: 10.1016/s0169-5002(02)00357-4. Lung Cancer. 2002. PMID: 12431829 Review.
-
Optimizing chemotherapy for advanced non-small cell lung cancer: focus on docetaxel.Lung Cancer. 2005 Dec;50 Suppl 2:S3-8. Lung Cancer. 2005. PMID: 16557668 Review.
Cited by
-
Single-cell RNA-sequencing uncovers compound kushen injection synergistically improves the efficacy of chemotherapy by modulating the tumor environment of breast cancer.Front Immunol. 2022 Oct 31;13:965342. doi: 10.3389/fimmu.2022.965342. eCollection 2022. Front Immunol. 2022. PMID: 36389835 Free PMC article.
-
Tailoring traditional Chinese medicine in cancer therapy.Mol Cancer. 2025 Jan 21;24(1):27. doi: 10.1186/s12943-024-02213-6. Mol Cancer. 2025. PMID: 39838407 Free PMC article. Review.
-
Uncovering the Anticancer Mechanism of Compound Sophorae Decoction against Ulcerative Colitis-Related Colorectal Cancer in Mice.Evid Based Complement Alternat Med. 2019 Oct 20;2019:8128170. doi: 10.1155/2019/8128170. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31772601 Free PMC article.
-
A New Strategy for Identifying Mechanisms of Drug-drug Interaction Using Transcriptome Analysis: Compound Kushen Injection as a Proof of Principle.Sci Rep. 2019 Nov 4;9(1):15889. doi: 10.1038/s41598-019-52375-3. Sci Rep. 2019. PMID: 31685921 Free PMC article.
-
Anti-Tumor Activities of Bioactive Phytochemicals in Sophora flavescens for Breast Cancer.Cancer Manag Res. 2020 Feb 27;12:1457-1467. doi: 10.2147/CMAR.S243127. eCollection 2020. Cancer Manag Res. 2020. PMID: 32161498 Free PMC article. Review.
References
-
- Ries LAG, Eisner MP, Kosary CL. SEER Cancer Statistics Review, 1975–2000. Bethesda: National Cancer Institute; 2003.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical