Sweet complementarity: the functional pairing of glycans with lectins
- PMID: 26956894
- PMCID: PMC11108359
- DOI: 10.1007/s00018-016-2163-8
Sweet complementarity: the functional pairing of glycans with lectins
Abstract
Carbohydrates establish the third alphabet of life. As part of cellular glycoconjugates, the glycans generate a multitude of signals in a minimum of space. The presence of distinct glycotopes and the glycome diversity are mapped by sugar receptors (antibodies and lectins). Endogenous (tissue) lectins can read the sugar-encoded information and translate it into functional aspects of cell sociology. Illustrated by instructive examples, each glycan has its own ligand properties. Lectins with different folds can converge to target the same epitope, while intrafamily diversification enables functional cooperation and antagonism. The emerging evidence for the concept of a network calls for a detailed fingerprinting. Due to the high degree of plasticity and dynamics of the display of genes for lectins the validity of extrapolations between different organisms of the phylogenetic tree yet is inevitably limited.
Keywords: Agglutinin; Antibodies; Glycobiology; Glycome; Lectins; Sialylation; Sugar code.
Figures
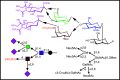
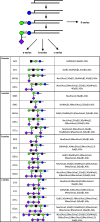
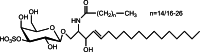
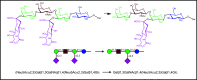




References
-
- Sharon N (1975) Complex Carbohydrates. Their Chemistry, Biosynthesis, and Functions. Addison-Wesley Publ. Co., Reading, MA, USA
-
- Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type I. J Exp Med. 1944;79(2):137–158. doi: 10.1084/jem.79.2.137. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

