MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration
- PMID: 26956930
- PMCID: PMC4786880
- DOI: 10.1038/ncomms10955
MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration
Abstract
Mitochondrial Ca(2+) uptake through the recently discovered Mitochondrial Calcium Uniporter (MCU) is controlled by its gatekeeper Mitochondrial Calcium Uptake 1 (MICU1). However, the physiological and pathological role of MICU1 remains unclear. Here we show that MICU1 is vital for adaptation to postnatal life and for tissue repair after injury. MICU1 knockout is perinatally lethal in mice without causing gross anatomical defects. We used liver regeneration after partial hepatectomy as a physiological stress response model. Upon MICU1 loss, early priming is unaffected, but the pro-inflammatory phase does not resolve and liver regeneration fails, with impaired cell cycle entry and extensive necrosis. Ca(2+) overload-induced mitochondrial permeability transition pore (PTP) opening is accelerated in MICU1-deficient hepatocytes. PTP inhibition prevents necrosis and rescues regeneration. Thus, our study identifies an unanticipated dependence of liver regeneration on MICU1 and highlights the importance of regulating MCU under stress conditions when the risk of Ca(2+) overload is elevated.
Figures
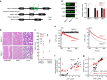

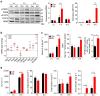
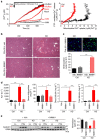
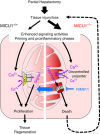
Comment in
-
Mitochondrial MIsCUes in liver regeneration.Hepatology. 2016 Nov;64(5):1797-1799. doi: 10.1002/hep.28816. Epub 2016 Sep 30. Hepatology. 2016. PMID: 27629287 No abstract available.
References
-
- Rizzuto R., De Stefani D., Raffaello A. & Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell. Biol. 13, 566–578 (2012). - PubMed
-
- Szabadkai G. & Duchen M. R. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 23, 84–94 (2008). - PubMed
-
- Kirichok Y., Krapivinsky G. & Clapham D. E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 (2004). - PubMed
-
- Gunter T. E. & Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258, C755–C786 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

