Blue light-dependent changes in loosely bound calcium in Arabidopsis mesophyll cells: an X-ray microanalysis study
- PMID: 26957564
- PMCID: PMC4915525
- DOI: 10.1093/jxb/erw089
Blue light-dependent changes in loosely bound calcium in Arabidopsis mesophyll cells: an X-ray microanalysis study
Erratum in
-
Corrigendum: Blue light-dependent changes in loosely bound calcium in Arabidopsis mesophyll cells: an X-ray microanalysis study.J Exp Bot. 2018 Apr 9;69(8):2171. doi: 10.1093/jxb/ery007. J Exp Bot. 2018. PMID: 29462465 Free PMC article. No abstract available.
Abstract
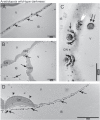
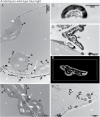
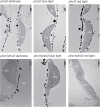
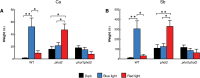
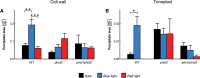
Calcium is involved in the signal transduction pathway from phototropins, the blue light photoreceptor kinases which mediate chloroplast movements. The chloroplast accumulation response in low light is controlled by both phot1 and phot2, while only phot2 is involved in avoidance movement induced by strong light. Phototropins elevate cytosolic Ca(2+) after activation by blue light. In higher plants, both types of chloroplast responses depend on Ca(2+), and internal calcium stores seem to be crucial for these processes. Yet, the calcium signatures generated after the perception of blue light by phototropins are not well understood. To characterize the localization of calcium in Arabidopsis mesophyll cells, loosely bound (exchangeable) Ca(2+) was precipitated with potassium pyroantimonate and analyzed by transmission electron microscopy followed by energy-dispersive X-ray microanalysis. In dark-adapted wild-type Arabidopsis leaves, calcium precipitates were observed at the cell wall, where they formed spherical structures. After strong blue light irradiation, calcium at the apoplast prevailed, and bigger, multilayer precipitates were found. Spherical calcium precipitates were also detected at the tonoplast. After red light treatment as a control, the precipitates at the cell wall were smaller and less numerous. In the phot2 and phot1phot2 mutants, calcium patterns were different from those of wild-type plants. In both mutants, no elevation of calcium after blue light treatment was observed at the cell periphery (including the cell wall and a fragment of cytoplasm). This result confirms the involvement of phototropin2 in the regulation of Ca(2+) homeostasis in mesophyll cells.
Keywords: Arabidopsis; blue light; calcium signaling; chloroplast movements; mesophyll cells; phototropin2.; thaliana.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Similar articles
-
Phosphoinositides play differential roles in regulating phototropin1- and phototropin2-mediated chloroplast movements in Arabidopsis.PLoS One. 2013;8(2):e55393. doi: 10.1371/journal.pone.0055393. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405144 Free PMC article.
-
Abscisic acid and blue light signaling pathways in chloroplast movements in Arabidopsis mesophyll.Acta Biochim Pol. 2016;63(3):449-58. doi: 10.18388/abp.2016_1382. Epub 2016 Aug 2. Acta Biochim Pol. 2016. PMID: 27486921
-
Decoding the role of phosphoinositides in phototropin signaling involved in chloroplast movements.Plant Signal Behav. 2013 Aug;8(8):e25105. doi: 10.4161/psb.25105. Epub 2013 Jun 3. Plant Signal Behav. 2013. PMID: 23733070 Free PMC article. Review.
-
Phototropin2 Contributes to the Chloroplast Avoidance Response at the Chloroplast-Plasma Membrane Interface.Plant Physiol. 2020 May;183(1):304-316. doi: 10.1104/pp.20.00059. Epub 2020 Mar 19. Plant Physiol. 2020. PMID: 32193212 Free PMC article.
-
Molecular insights into the phototropin control of chloroplast movements.J Exp Bot. 2022 Oct 18;73(18):6034-6051. doi: 10.1093/jxb/erac271. J Exp Bot. 2022. PMID: 35781490 Review.
Cited by
-
Genetic architecture of a light-temperature coincidence detector.Nat Commun. 2025 Aug 26;16(1):7947. doi: 10.1038/s41467-025-62194-y. Nat Commun. 2025. PMID: 40858539 Free PMC article.
-
The involvement of cyclotides in the heavy metal tolerance of Viola spp.Sci Rep. 2024 Aug 20;14(1):19306. doi: 10.1038/s41598-024-69018-x. Sci Rep. 2024. PMID: 39164283 Free PMC article.
-
Calcium Positively Mediates Blue Light-Induced Anthocyanin Accumulation in Hypocotyl of Soybean Sprouts.Front Plant Sci. 2021 May 28;12:662091. doi: 10.3389/fpls.2021.662091. eCollection 2021. Front Plant Sci. 2021. PMID: 34122484 Free PMC article.
-
Calcium changes in Robinia pseudoacacia pulvinar motor cells during nyctinastic closure mediated by phytochromes.Protoplasma. 2019 May;256(3):615-629. doi: 10.1007/s00709-018-1323-0. Epub 2018 Oct 31. Protoplasma. 2019. PMID: 30382423
-
Effects of catalase on chloroplast arrangement in Opuntia streptacantha chlorenchyma cells under salt stress.Sci Rep. 2017 Aug 17;7(1):8656. doi: 10.1038/s41598-017-08744-x. Sci Rep. 2017. PMID: 28819160 Free PMC article.
References
-
- An Q, Hückelhoven R, Kogel KH, Van Bel AJE. 2006. Multivesicular bodies participate in a cell wall-associated defense response in barley leaves attacked by the pathogenic powdery mildew fungus. Cellular Microbiology 8, 1009–1019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

