Fully reduced granulin-B is intrinsically disordered and displays concentration-dependent dynamics
- PMID: 26957645
- PMCID: PMC4830411
- DOI: 10.1093/protein/gzw005
Fully reduced granulin-B is intrinsically disordered and displays concentration-dependent dynamics
Abstract
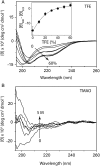
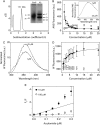
Granulins (Grns) are a family of small, cysteine-rich proteins that are generated upon proteolytic cleavage of their precursor, progranulin (Pgrn). All seven Grns (A-G) contain 12 conserved cysteines that form 6 intramolecular disulfide bonds, rendering this family of proteins unique. Grns are known to play multi-functional roles, including wound healing, embryonic growth, and inflammation and are implicated in neurodegenerative diseases. Despite their manifold functions, there exists a dearth of information regarding their structure-function relationship. Here, we sought to establish the role of disulfide bonds in promoting structure by investigating the fully reduced GrnB (rGrnB). We report that monomeric rGrnB is an intrinsically disordered protein (IDP) at low concentrations. rGrnB undergoes dimerization at higher concentrations to form a fuzzy complex without a net gain in the structure-a behavior increasingly identified as a hallmark of some IDPs. Interestingly, we show that rGrnB is also able to activate NF-κB in human neuroblastoma cells in a concentration-dependent manner. This activation correlates with the observed monomer-dimer dynamics. Collectively, the presented data establish that the intrinsic disorder of rGrnB governs conformational dynamics within the reduced form of the protein, and suggest that the overall structure of Grns could be entirely dictated by disulfide bonds.
Keywords: cysteine-rich protein; fuzzy complex; granulin; intrinsically disordered protein; progranulin.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Abràmoff M.D., Magalhães P.J., Ram S.J. (2004) Biophotonics Int., 11, 36–43.
-
- Baker M., Mackenzie I.R., Pickering-Brown S.M. et al. (2006) Nature, 442, 916–919. - PubMed
-
- Baldwin A.S. (1996) Annu. Rev. Immunol., 14, 649–681. - PubMed
-
- Bateman A., Bennett H.P. (1998) J. Endocrinol., 158, 145–151. - PubMed
-
- Boteva R., Zlateva T., Dorovska-Taran V., Visser A.J., Tsanev R., Salvato B. (1996) Biochemistry, 35, 14825–14830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

