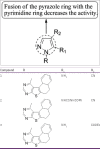
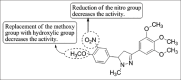
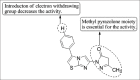
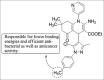
Current status of pyrazole and its biological activities
- PMID: 26957862
- PMCID: PMC4766773
- DOI: 10.4103/0975-7406.171694
Current status of pyrazole and its biological activities
Abstract
Pyrazole are potent medicinal scaffolds and exhibit a full spectrum of biological activities. This review throws light on the detailed synthetic approaches which have been applied for the synthesis of pyrazole. This has been followed by an in depth analysis of the pyrazole with respect to their medical significance. This follow-up may help the medicinal chemists to generate new leads possessing pyrazole nucleus with high efficacy.
Keywords: Anti-microbial; hetrocyclic and biological activity; pyrazole.
Figures

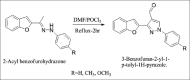
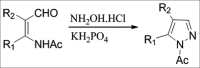
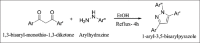
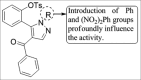
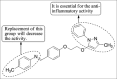
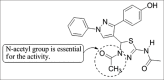
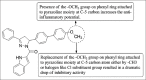
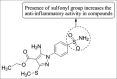
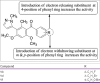
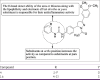
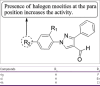

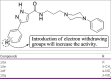
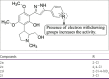
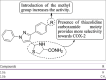
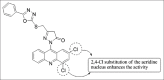
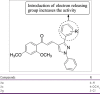


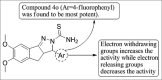
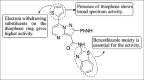
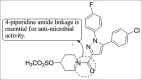
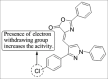
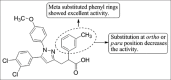
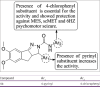
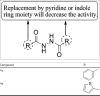
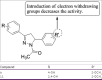


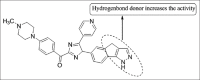
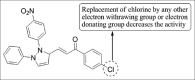
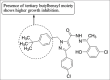
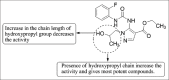
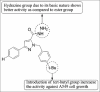
References
-
- Perrin DD. London: Butterworths; 1972. Dissociation Constants of Organic Bases in Aqueous Solution.
-
- Eicher T, Hauptmann S. 2nd ed. New York: Wiley-VCH; 2003. The Chemistry of Heterocycles: Structure, Reactions, Synthesis and Applications.
-
- Katz AM, Pearson CM, Kennedy JM. A clinical trial of indomethacin in rheumatoid arthritis. Clin Pharmacol Ther. 1965;6:25–30. - PubMed
-
- Ilango K, Valentina P. 1st ed. India: Keerthi Publishers; 2007. Textbook of Medicinal Chemistry; pp. 327–33.
-
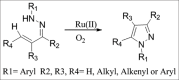
- Panda N, Jena AK. Fe-catalyzed one-pot synthesis of 1,3-di- and 1,3,5-trisubstituted pyrazoles from hydrazones and vicinal diols. J Org Chem. 2012;77:9401–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

